NCERT Exemplar Class 12 Chemistry Chapter 13 Aminese are part of NCERT Exemplar Class 12 Chemistry . Here we have given NCERT Exemplar Class 12 Chemistry Chapter 13 Amines.
NCERT Exemplar Class 12 Chemistry Chapter 13 Amines
Multiple Choice Questions
Single Correct Answer Type
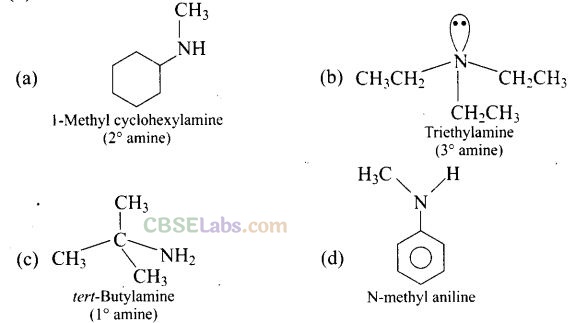
Question 1. Which of the following is a 3° amine?
(a) 1 -Methylcyclohexylamine (c) tert-Butylamine
Solution:
(b)
Question 2. The correct IUPAC name for CH
2
= CHCH
2
NHCH
3
is
(a) allylmethylamine (b) 2-amino-4-pentene
(c) 4-aminopent-l-ene . (d) N-methylprop-2-en-l-amine.
Solution:
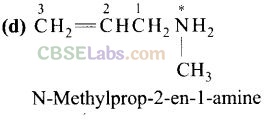
Question 3. Amongst the following, the strongest base in aqueous medium is .
(a) CH
3
NH
2
(b) NCCH
2
NH
2
(C) (CH
3
)
2
NH (d) C
6
H
5
NHCH
3
Solution:
(c) 2° amine is more basic than 1° amine, i.e., (CH
3
)
2
NH is more basic than CH
3
NH
2
. Due to -I effect of CN group, NC-CH
2
NH
2
is less basic than CH
3
NH
2
. Further C
6
H
5
NHCH
3
is less basic than both CH
3
NH
2
and (CH
3
)
2
NH due to delocalization of lone pair of electrons present on the nitrogen atom into benzene ring. Hence, the decreasing order of amines is:
(CH
3
)
2
NH > CH
3
NH
2
> C
6
H
5
NHCH
3
> NC – CH
2
NH
2
Question 4. Which of the following is the weakest Bronsted base?
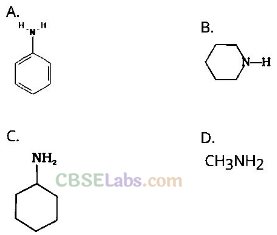
Solution:
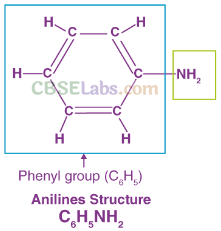
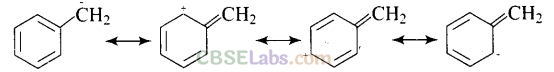
(a) Due to delocalization of lone pair of electrons on the N-atom into the benzene ring, C6H5NH2 is the weakest base.
Resonating Structure of Aniline
Question 5. Benzylamine may be alkylated as shown in the following equation: C
6
H
5
CH
2
NH
2
+ R – X > C
6
H
5
CH
2
NHR
Which of the following alkyl halides is best suited for this reaction through SN1 mechanism? .
(a) CH
3
Br (b) C
6
H
5
Br (c) C
6
H
5
CH
2
Br (d) C
2
H
5
Br
Solution:
(c) SN1 reaction occurs in two steps. In first step R – X bond is broken to produce a carbocation which is attacked by nucleophile. The greater the stability of carbocation, the greater will be the rate of reaction. Benzylic halides show high reactivity towards SN1 reaction.
Question 6. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine?
(a) H
2
(excess)/Pt (b) LiAlH
4
in ether
(c) Fe and HCl (d) Sn and HCl
Solution:
(b) Aryl nitro compound cannot be converted into amine using LiAlH4 in ether.

Question 7. In order to prepare a 10 amine‘from an alkyl halide with simultaneous addition of one CH
2
group in the carbon chain, the reagent used as source of nitrogen is
(a) sodium amide, NaNH
2
(b) sodium azide, NaN
2
(c) potassium cyanide, KCN
(d) potassium phthalimide, C
6
H
4
(CO
2
)N K
Solution:
(c) KCN is used to increase number of carbon atoms.

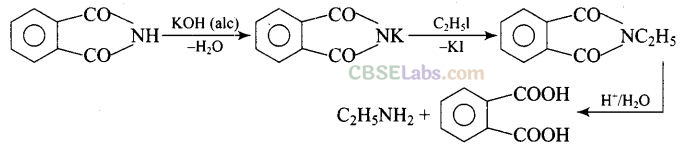
Question 8. The source of nitrogen in Gabriel synthesis of amines is .
(a) sodium azide, NaN
3
(b) sodium nitrite, NaNO
2
(c) potassium cyanide, KCN
(d) potassium phthalimide, C
6
H
4
(CO
2
)N’KT
Solution:
(d) Potassium phthalimide is the source of nitrogen in Gabriel’s synthesis.
Question 9. Amongst the given set of reactants, the most appropriate for preparing 2° amine is .
(a) 2° R – Br + NH
3
(b) 2° R – Br + NaCN followed by H
2
/Pt
(c) 10 R – NH
2
+ RCHO followed by H
2
/Pt
(d) 1° R – Br + (2 mol) + potassium phthalimide followed by H
3
O+/heat
Solution:
(c)
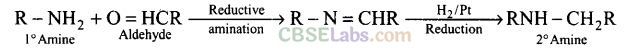
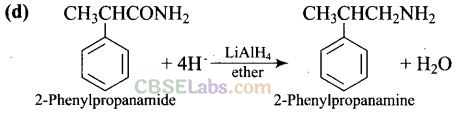
Question 10. The best reagent for converting 2-phenylpro-panamide into-2-phenyl- propanamine is .
(a) excess H
2
(b) Br
2
in aqueous NaOH
(c) iodine in the presence of red phosphorus
(d) LiAlH
4
in ether
Solution:
Question 11. The best reagent for converting-2-phenylpro-panamide into 1-phenylethana- mine is .
(a) excess H
2
/Pt (b) NaOH /Br
2
(c) NaBH
4
/methanol (d) LiAlH
4
/ether
Solution:
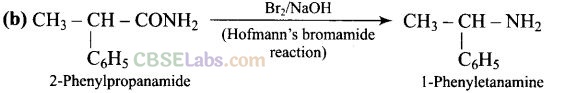
Question 12. Hoffmann bromamide degradation reaction is shown by .
(a) ArNH
2
(b) ArCONH
2
(c) ArNO
2
(d) ArCH
2
NH
2
Solution:
![]()
Question 13. The correct increasing order of basic strength for the following compounds is
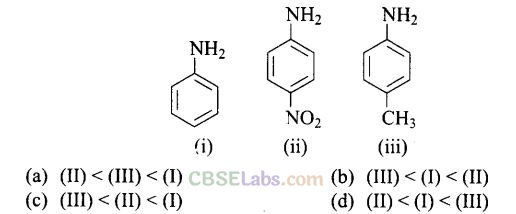
Solution:
(d)
Electron withdrawing group decreases the basic strength while electron releasing groups increases the basic strength of aniline.
Question 14. Methylamine reacts with HN03 to form .
(a) CH3-0-N = 0 (b) CH3-0-CH3
(c) CH3OH (d) CH3CHO
Solution:
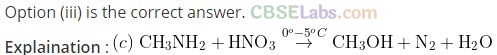
Question 15. The gas evolved when methylamine reacts with nitrous acid is .
(a) NH
3
(b) N
2
(C) H
2
(d) C
2
H
6
Solution:
(b) Chemical reaction takes place during reaction of methylamine with nitrous acid is as follows
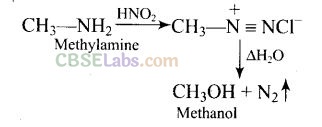
Question 16. In the nitration of benzene using a mixture of cone. H
2
SO
4
and cone. HNO
3
,
the species which initiates the reaction is .
(a) NO
2
(b) NCf (c) NO
2
(d) NH
2
Solution:
(c)
NO
2
(Nitronium ion) electrophile initiates the process of nitration. It is obtained as:
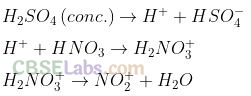
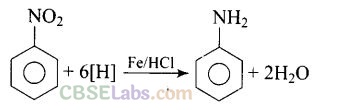
Question 17. Reduction of aromatic nitro compounds using Fe and HCl gives .
(a) aromatic oxime (b) aromatic hydrocarbon
(c) aromatic primary amine (d) aromatic amide
Solution:
(c)
Question 18. The most reactive amine towards dilute hydrochloric acid is
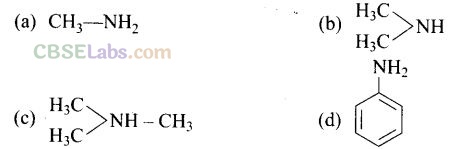
Solution:
(b) The greater will be the strength of base, the greater will be its reactivity towards dilute HCl. Hence, (CH
3
)
2
NH has the highest basic strength as it has the highest reactivity.
Question 19. Acid anhydrides on reaction with primary amines give .
(a) amide (b) imide
(c) secondary amine (d) imine
Solution:
![]()
Question 20.
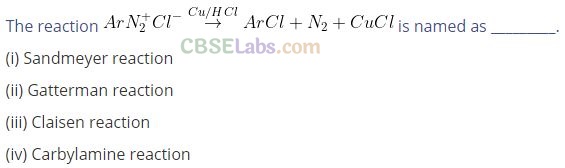
Solution:
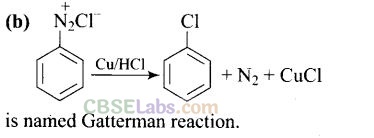
Question 21. Best method for preparing primary amines from alkyl halides without
changing the number of carbon atoms in the chain is
(a) Hoffmann bromamide reaction (b) Gabriel phthalimide reaction (c) Sandmeyer reaction (d) reaction with NH
3
Solution:
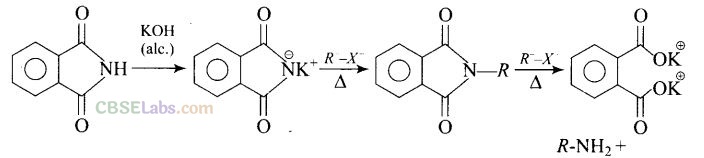
(b) Gabriel phthalimide synthesis is used to get primary amine from alkyl halide without any change in number of carbon atoms.
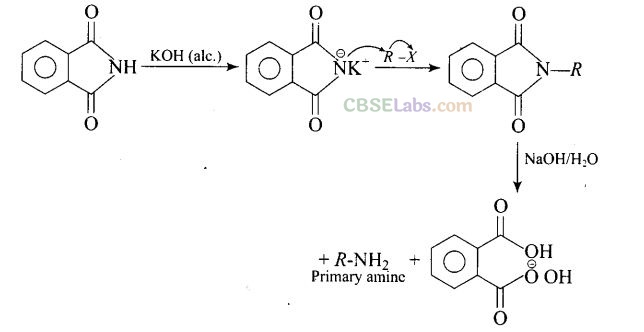
Question 22. Which of the following compound will not undergo azo coupling reaction with benzene diazonium chloride?
(a) Aniline (b) Phenol (c) Anisole (d) Nitrobenzene
Solution:
(d) Diazonium cation is a weak electrophile and hence reacts with electron rich compounds containing electron donating groups such as -OH, -NH
2
and -OCH
3
groups and not with compounds containing electron withdrawing groups such as -NO
2
, etc.
Question 23. Which of the following compounds is the weakest Bronsted base?

Solution:
(c) Amines (a, b) have stronger tendency to accept a proton and hence are stronger Bronsted bases than phenol (c) and alcohol (d). Since phenol is more acidic than alcohol, therefore, phenol (c) has the least tendency to accept a proton and hence it is the weakest Bronsted base.
Question 24. Among the following amines’ the strongest Bronsted base is

Solution:
(d) Aniline is weaker base than NH
3
due to delocalization of lone pair of electrons on the N-atom into the benzene ring. Pyrrole (c) is not at all basic because the lone pair of electrons on N-atom is donated towards aromatic sextet formation. Therefore, pyrrolidine (d) has a strong tendency to accept a proton and is hence, the strongest base.
Question 25. The correct decreasing order of basic strength of the following species is
H
2
O, NH
3
, OH , NH;
(a) NH
3
> OH > NH
3
> H
2
O (b) OH > NH
2
> H
2
O > NH
3
(c) NH
3
> H
2
O > NH
2
> OH (d) H
2
O > NH
3
> OH > NH
2
Solution:
(a) NH
2
> OH > NH
3
> H
2
O. Due to higher electronegativity of O than N atom, O – H bond is more polar than N – H bond. Hence, O – H is more acidic in nature than N – H bond. Now, NH
2
and OH have negative charge due to which they are more basic than NH3 and H20.
Question 26. Which of the following should be most volatile?
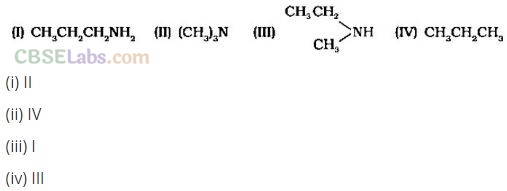
Solution:
(b) 1° and 2° amines have higher boiling points due to intermolecular H-bonding (and hence less volatile than 3° amines and hydrocarbons of comparable molecular mass).
Question 27. Which of the following methods of preparation of amines will not give same number of carbon atoms in the chain of amines as in the reactant?
(a) Reaction of nitrile with LiAlH4
(b) Reaction of amide with LiAlH4 followed by treatment with water.
(c) Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis
(d) Treatment of amide with bromine in aqueous solution of sodium hydroxide.
Solution:
(d) Aliphatic and arylalkyl primary amines can be prepared by the reduction of the corresponding nitriles with LiAlH4.
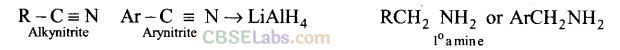
Heating alkyl halide with primary, secondary and tertiary amine can be prepared by reduction of LiAlH4 followed by treatment with water.
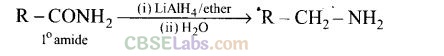
Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis produces primary amine. This process is known as Gabriel phthalimide reaction. The number of carbon atoms in the chain of amines of product is same as reactant.
More than One Correct Answer Type
Question 28. Which of the following cannot be prepared by Sandmeyer’s reaction?
(a) Chlorobenzene (b) Bromobenzene
(c) Iodobenzene (d) Fluorobenzene
Solution:
(c, d) Chloro and bromo arenes are easily prepared by Sandmeyer’s reaction. Iodoarenes are prepared by simply warming the diazonium salt solution with aqueous KI solution.
Fluoroarenes are prepared by Balz-Schiemann reaction.
All other reagents give aniline.
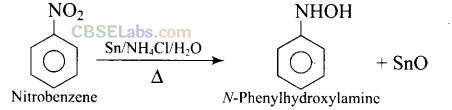
Question 29. Reduction of nitrobenzene by which of the following reagents give aniline?
(a) Sn/HCl (b) Fe/HCl (c) H
2
-Pd (d) Sn/NH
4
OH
Solution:
(a, b, c)
Question 30. Which of the following species are involved in the carbylamine test?
(a) R-NC (b) CHCl
3
(c) COCl
2
(d) NaN0
2
+ HCl
Solution:
(a, b) Carbylamine reaction: Amine on reaction with a mixture of CHCl
3
and KOH produces alkyl isocyanate.
R-NH
2
+ CHCl, + 3KOH -> RNC + 3KCl + 3H
2
O Only RNC and CHCl
3
are involved in carbylamine reaction.
Question 31. The reagents that can be used to convert benzene diazonium chloride to
benzene are
(a) SnCl
2
/HCl (b) CH
3
CH
2
OH
(c) H
3
PO
2
(d) LiAlH
4
Solution:
(a, b)
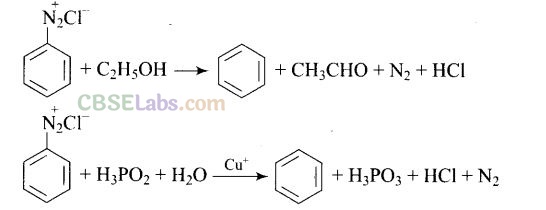
Question 32. The Product of the following reaction is .
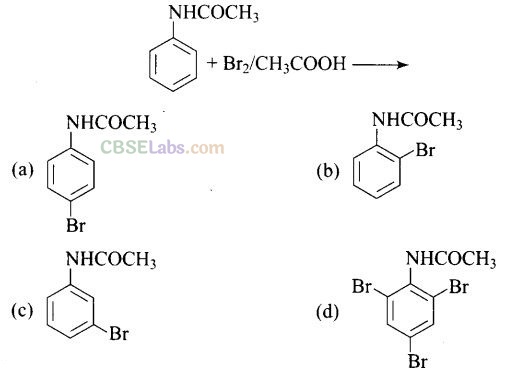
Solution:
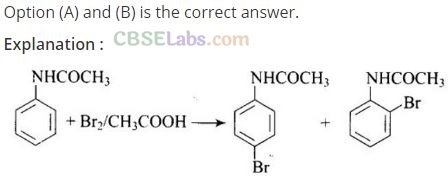
Question 33. Arenium ion involved in the bromination of aniline is.
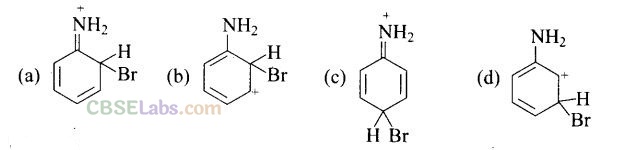
Solution:
(a,b,c)
Arenium ion involved in the bromination of aniline are as follows
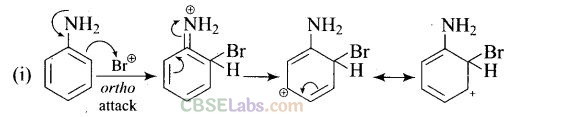
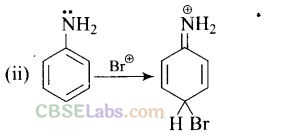
Question 34. Which of the following amines can be prepared by Gabriel synthesis?
(a) Isobutyl amine (b) 2-Phenylethylamine
(c) N-Methylbenzylamine (d) Aniline
Solution:
(a, b)
Only primary aliphatic amines such as (a) (CH
3
)
2
CH-CH
2
NH
2
and C
6
H
5
CH
2
NH
2
(b) can be prepared by Gabriel synthesis. 2° amines, i.e., C
6
H
5
CH
2
NHCH
3
(C) and 1° amine, C
6
H
5
NH
2
(d), however, cannot be prepared.
Question 35. Which of the following reactions are correct?
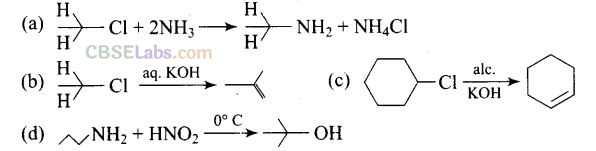
Solution:
CH3CH2NH2 + NH4C1
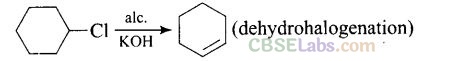
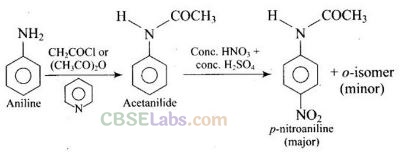
Question 36. Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(a) Acetyl chloride/pyridine followed by reaction with cone. H
2
SO
4
+ cone.
(b) Acetic anhydride/pyridine followed by cone. H
2
SO
4
+ cone. HNO
3
(c) Dil. HCl followed by reaction with cone. H
2
SO
4
+ cone. HNO
3
(d) Reaction with cone. HNO
3
+ cone. H
2
S0
4
Solution:
(a, b)
Aniline or reaction with acetyl chloride or acetic anhydride in the presence of pyridine produces N-acetyl aniline which is a ortho, para directing group which on further reaction with nitrating mixture (cone. HN0
3
+ cone. H2SO
4
) produces p-nitroaniline preferentially as shown below:
Question 37. Which of the following reactions belong to electrophilic aromatic substitution?
(a) Bromination of acetanilide
(b) Coupling reaction of aryldiazonium salts
(c) Diazotisation of aniline
(d) Acylation of aniline
Solution:
(a, b) Acylation is a nucleophilic substitution reaction in which H atom of -NH
2
is replaced by acyl group. Diazotisation is also a nucleophilic substitution reaction.
Short Answer Type Questions
Question 38. Which is the role of HNO
3
in the nitrating mixture used for nitration of
benzene?
Solution:
HNO
3
acts as a base in the nitrating mixture (HNO
3
+ H
2
SO
4
) and provides the electrophile.
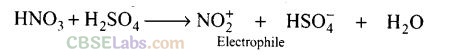
Question 39. Why is NH
2
group of aniline acetylated before carrying out nitration?
Solution:
Direct nitration of aniline is not possible on account of oxidation of -NH
2
group. However, nitration can be carried after protecting the -NH
2
group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.
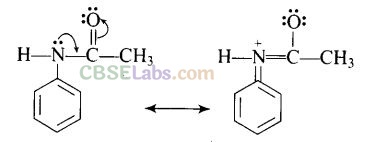
As a result, the activating effect -NH
2
group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of —NHCOCH
3
group is less than that of —NH
2
group.
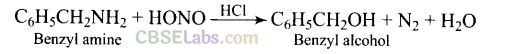
Question 40. What is the product when C
6
H
5
CH
2
NH
2
reacts with HN02?
Solution:
Question 41. What is the best reagent to convert nitrile to primary amine?
Solution:
Reduction of nitriles with sodium alcohol or LiAlH
4
gives primary amine.

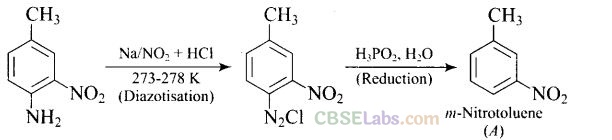
Question 42. Give the structure of ‘A’ in the following reaction.
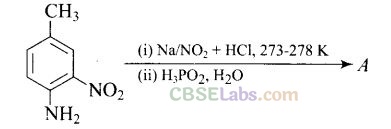
Solution:
Question 43. What is Hinsberg reagent?
Solution:
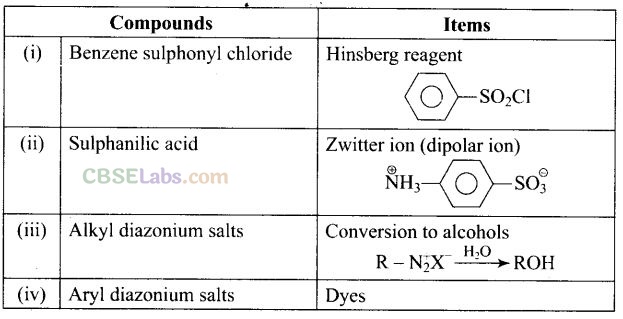
Hinsberg reagent is benzene sulphonyl chloride (C
6
H
5
SO
2
Cl).
Question 44. Why is benzene diazonium chloride not stored and is used immediately after its preparation?
Solution:
Benzene diazonium chloride is very unstable. Therefore, it cannot be stored and is used immediately after its preparation.
Question 45. Why does acetylation of -NH
2
group of aniline reduce its activating effect?
Solution:
Direct nitration of aniline is not possible on account of oxidation of -NH
2
group. However, nitration can be carried after protecting the -NH
2
group by acetylation to give acetanilide which is then nitrated and finally hydrolysed to give o- and p-nitroanilines.
The acetyl group being electron withdrawing attracts the lone pair of electrons of the N-atom towards carbonyl group.
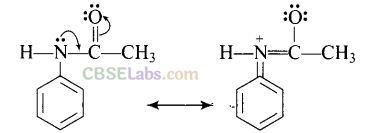
As a result, the activating effect -NH
2
group is reduced i.e., the lone pair of electrons on nitrogen is less available for donation to benzene ring by resonance. Therefore, activating effect of —NHCOCH
3
group is less than that of —NH
2
group.
Question 46. Explain why is MeNH
2
stronger base than MeOH?
Solution:
Nitrogen is less electronegative than oxygen, therefore, lone pair of electrons on nitrogen is readily available for donation. Hence, MeNH
2
is more basic than MeOH.
Question 47. What is the role of pyridine in the acylation reaction of amines?
Solution:
Pyridine and other bases are used to remove the side product i.e., HCl formed during reaction mixture.
Question 48. Under what reaction conditions (acidic/basic), the coupling reaction of aryl diazonium chloride with aniline is carried out?
Solution:
Coupling reaction of aryl diazonium chloride with aniline is carried out in mild acidic condition (pH = 4-5).
Question 49. Predict the product of reaction of aniline with bromine in non-polar solvent such as CS
2
.
Solution:
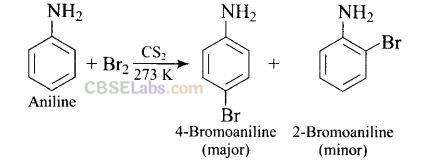
In non-polar solvent (such as CS
2
) the activating effect of -NH
2
group is reduced (due to resonance) and hence, mono substitution occurs only at o- and p-positions.
Question 50. Arrange the following compounds in increasing order of dipole moment: CH
3
CH
2
CH
3
, CH
3
CH
2
NH
2
, CH
3
CH
2
OH.
Solution:
CH
3
CH
2
CH
3
< CH
3
CH
2
NH
2
< CH
3
CH
2
OH
Since O is more electronegative than N, therefore, dipole moment of ethyl alcohol is higher than that of ethyl amine. Propane however, has the least dipole moment since it is almost a non-polar molecule.
Question 51. What is the structure and IUPAC name of the compound, allyl amine?
Solution:
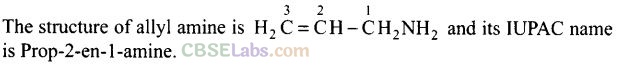
Question 52. Write down the IUPAC name of
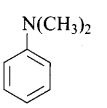
Solution:
N, N-dimethylbenzenamine.
Question 53. A compound Z with molecular formula C
3
H
9
N reacts with C
6
H
5
SO
2
Cl to give a solid, insoluble in alkali, identify Z.
Solution:
Compound ‘Z’ with molecular formula C
3
H
9
N is an aliphatic amine which on treatment with C
6
H
5
SO
2
Cl gives a solid, insoluble in alkali. Therefore, the product does not have any replaceable hydrogen on the nitrogen atom. In other words, the amines (Z) must be secondary amine, i.e., Z is ethyl methylamine (C
2
H
5
NHCH
3
).
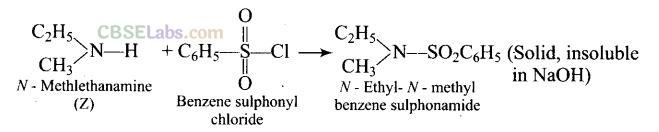
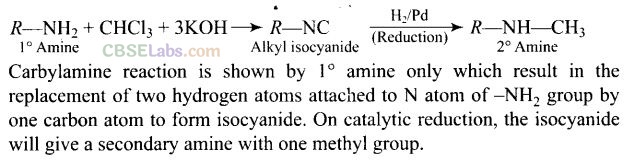
Question 54. A primary amine, RNH
2
can be reacted with CH
3
-X to get secondary amine, R NHCH
3
but the only disadvantage is that 3° amine and quaternary ammonium salts are also obtained as side products. Can you suggest a method where RNH
2
forms only 2° amine?
Solution:
Question 55. Complete the following reaction.
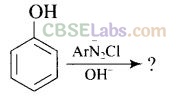
Solution:
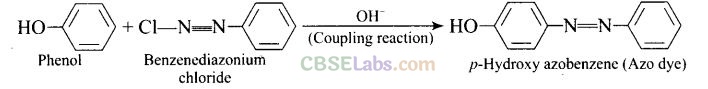
Question 56. Why is aniline soluble in aqueous HCl?
Solution:
Aniline forms anilinium chloride salt with aqueous HCl which is soluble in water.
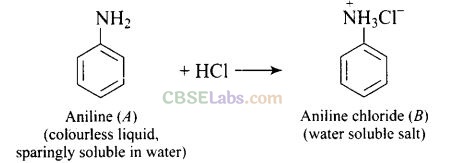
Question 57. Suggest a route by which the following conversion can be accomplished.
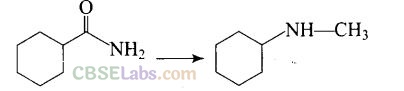
Solution:
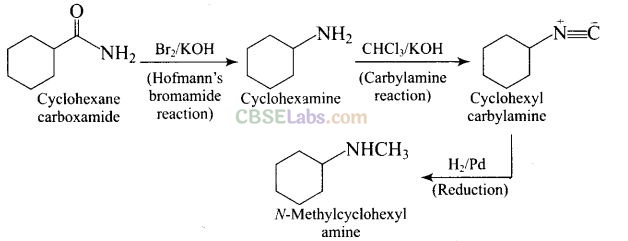
Question 58. Identify A and B in the following reaction.
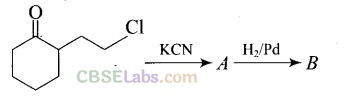
Solution:
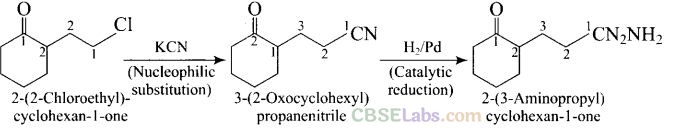
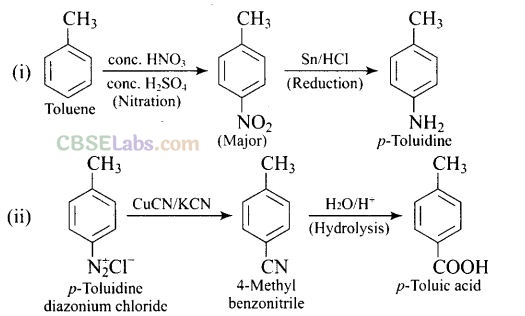
Question 59. How will you carry out the following conversions?
(i) toluene –> p-toIuidine
(ii) p-toluidine diazonium chloride –> p-toluic acid
Solution:
Question 60. Write following conversions:
(i) nitrobenzene –> acetanilide
(ii) acetanilide –> p-nitroaniline
Solution:
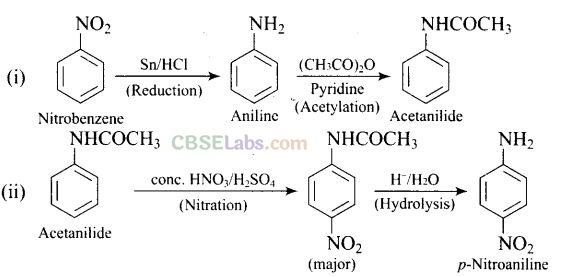
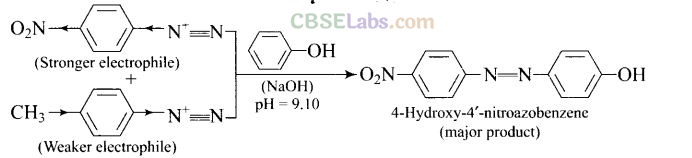
Question 61. A solution contains 1 g mol each of p-toluene diazonium chloride and p-nitrophenyl diazonium chloride. To this 1 g mol of alkaline solution of phenol is added. Predict the major product. Explain your answer.
Solution:
This reaction is an example of electrophilic aromatic substitution. In alkaline medium, phenol forms phenoxide ion which is more electron rich than phenol and hence more reactive for electrophilic attack. The electrophile in this reaction is arydiazonium cation. p-Nitrophenyldiazonium cation is a stronger electrophile than p-toluene diazonium cation. Therefore, it couples preferentially with phenol.
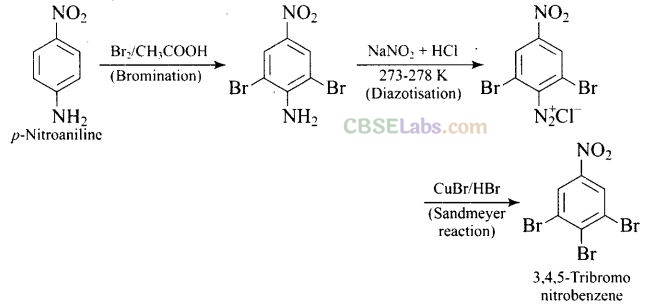
Question 62. How will you bring out the following conversion?
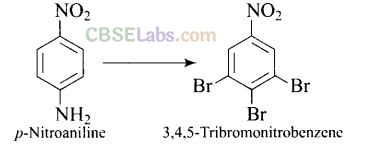
Solution:
Question 63. How will you carry out the following conversion?
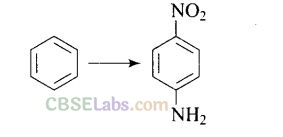
Solution:
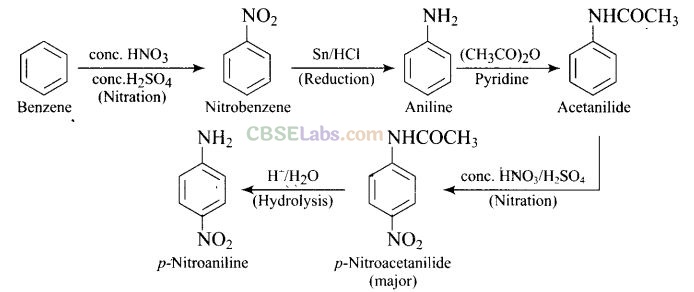
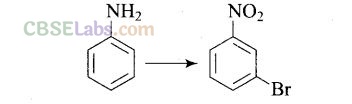
Question 64. How will you carry out the following conversion?
Solution:
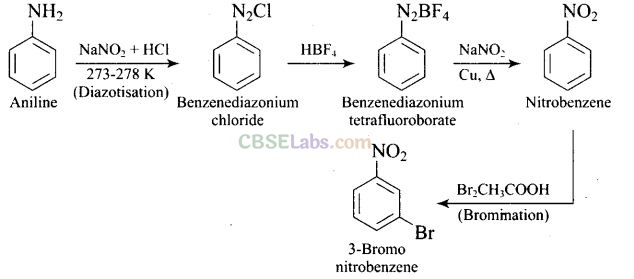
Question 65.How will you carry out the following conversion?
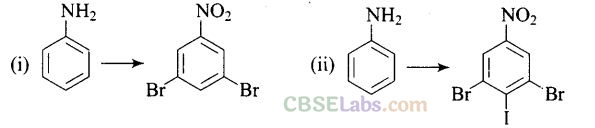
Solution:
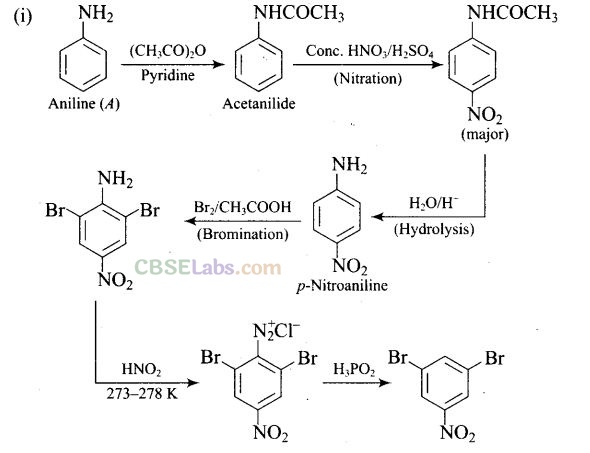
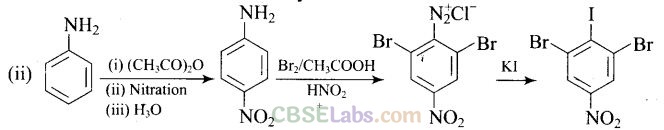
Matching Column Type
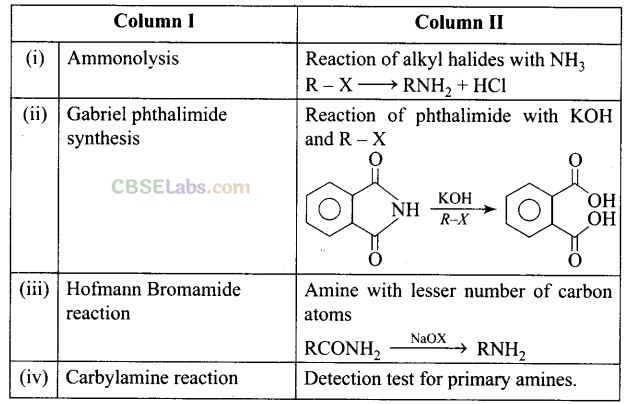
Question 66. Match the reactions given in Column 1 with the statements given in Column II.
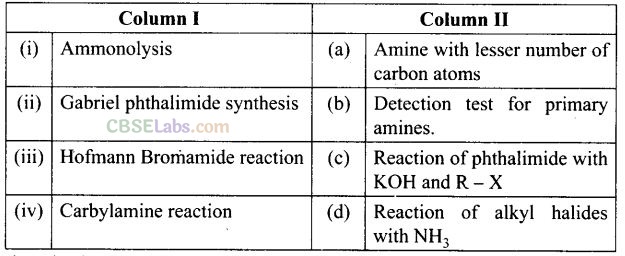
Solution:
(i —» d), (ii —»c), (iii -» a), (iv-> b)
Question 67. Match the compounds given in Column I with the items given in Column II.
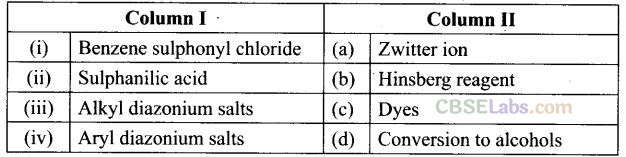
Solution:
(i -> b), (ii —» a), (iii —» d), (iv -> c)
Assertion and Reason Type Questions
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct answer out of the following choices:
(a) Both Assertion and Reason are wrong.
(b) Both Assertion and Reason are correct but Reason is not the correct explanation of Assertion.
(c) Assertion is correct but Reason is wrong.
(d) Both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
(e) Assertion is wrong but Reason is correct.
Question 68. Assertion (A): Acylation of amines gives a monosubstituted product, whereas alkylation of amines gives polysubstituted product.
Reason (R): Acyl group sterically hinders the approach of further acyl groups.
Solution:
(c) Amines on acetylation give monosubstituted product, while on alkylation gives polysubstitution product as well.
Question 69. Assertion (A): Hofmann’s bromamide reaction is given by primary amines. Reason (R): Primary amines are more basic than secondary amines.
Solution:
(a) Hofmann’s bromamide reaction is given by amides, not by amines. Moreover, primary amines are basic than secondary amines.
Question 70. Assertion (A): N-Ethylbenzene sulphonamide is soluble in alkali.
Reason (R): Hydrogen attached to nitrogen in sulphonamide is strongly acidic.
Solution:
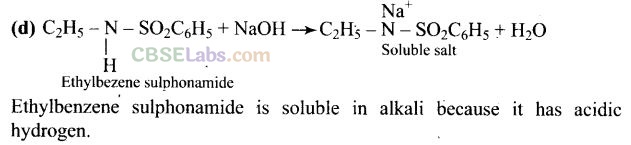
Question 71. Assertion (A): N, N-diethylbenzene sulphonamide is insoluble in alkalf. Reason (R): Sulphonyl group attached to nitrogen atom is strong electron withdrawing group.
Solution:
(b) N, N-diethyl benzene sulphonamide is insoluble in alkali because it has no acidic hydrogen.
Sulphonyl group attached to nitrogen atom is electron withdrawing group.
Question 72. Assertion (A): Only a small amount of HCl is required in the reduction of nitro compounds with iron scrap and HCl in the presence of steam.
Reason (R): FeCl
2
formed gets hydrolysed to release HCl during the reaction.
Solution:
(d) Fe + 2HCl >FeCl
2
+ 2[H]
Nascent hydrogen reduces nitro compounds.
FeCl
2
+ H
2
O(g) > FeO + 2HCl
Question 73. Assertion (A): Aromatic 1° amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
Solution:
(a) Aromatic amines are never obtained by Gabriel phthalimide synthesis.
Question 74. Assertion (A): Acetanilide is less basic than aniline.
Reason (R): Acetylation of aniline results in decrease of electron density on nitrogen.
Solution:
(d) Acetanilide is less basic than aniline because electron density of nitrogen is lowered by acetyl group.
Long Answer Type Questions
Question 75. A hydrocarbon ‘A’, (C
4
H
8
) on reaction with HC1 gives a compound ‘B'(C
4
H
9
Cl), which on reaction with 1 mol of NH
3
gives compound ‘C’, (C
4
H
11
N). On reacting with NaN02 and HC1 followed by treatment with water, compound ‘C’ yields an optically active alcohol, ‘D’. Ozonolysis of ‘A’ gives 2 moles of acetaldehyde. Identify compounds ‘A’ to ‘D’. Explain the reactions involved.
Solution:
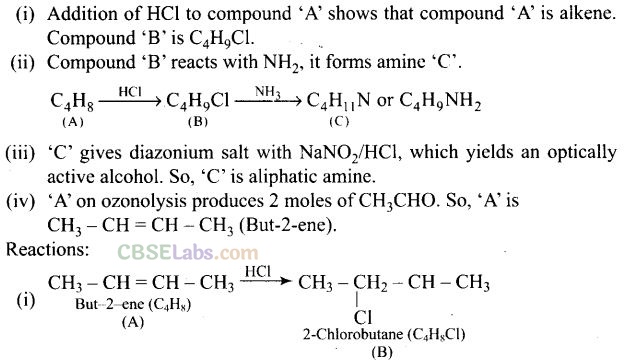
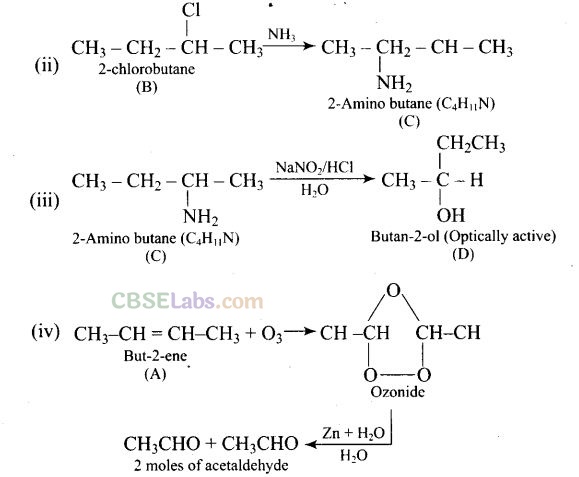
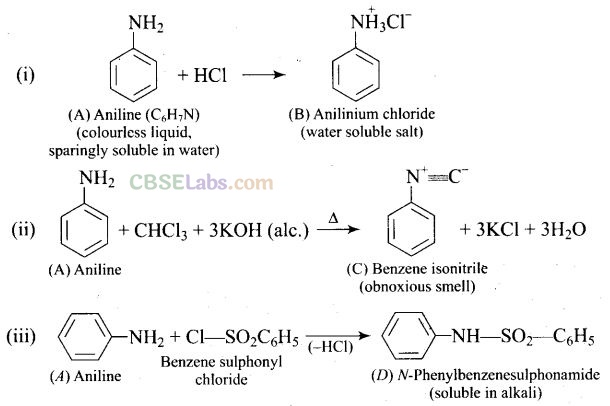
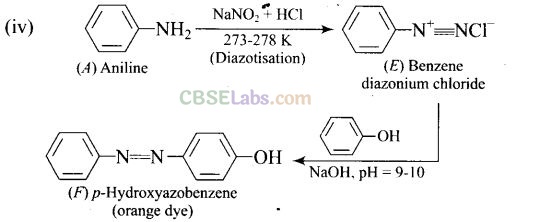
Question 76. A colourless substance ‘A’ (C
6
H
7
N) is sparingly soluble in water and gives a water soluble compound ‘B’ on treating with mineral acid. On reacting with CHCl
3
and alcoholic potash ‘A’ produces an obnoxious smell due to the formation of compound ‘C’. Reaction of ‘A’ with benzenesulphonyl chloride gives compound ‘D’ which is soluble in alkali. With NaNO
2
and HCl, ‘A’ forms compound ‘E’ which reacts with phenol in alkaline medium to give an orange dye ‘F’ Identify compounds ‘A’ to ‘F’
Solution:
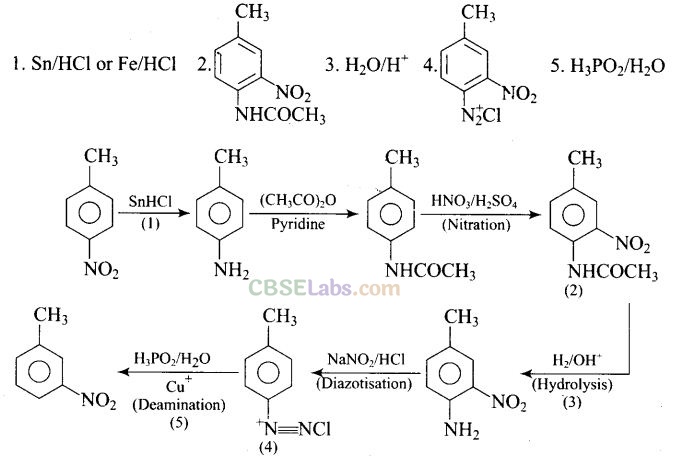
Question 77. Predict the reagent or the product in the following reaction sequence.
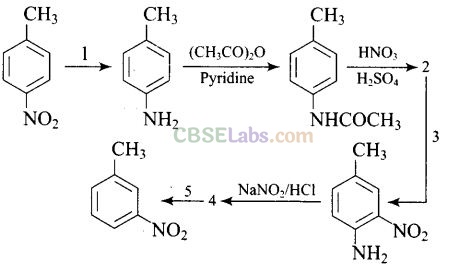
Solution:
NCERT Exemplar Class 12 Chemistry Solutions
- Chapter 1 Solid State
- Chapter 2 Solution
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p-Block Elements
- Chapter 8 The d- and f-Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes and Haloarenes
- Chapter 11 Alcohols, Phenols and Ethers
- Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life