NCERT Exemplar Class 11 Chemistry Chapter 2 Structure of Atom are part of NCERT Exemplar Class 11 Chemistry . Here we have given NCERT Exemplar Class 11 Chemistry Chapter 2 Structure of Atom.
NCERT Exemplar Class 11 Chemistry Chapter 2 Structure of Atom
Q1. Which of the following conclusions could not be derived from Rutherford’s α-particle scattering experiment?
(a) Most of the space in the atom is empty.
(b) The radius of the atom is about 10
-10
m while that of nucleus is 10
-15
(c) Electrons move in a circular path of fixed energy called orbits.
(d) Electrons and the nucleus are held together by electrostatic forces of attraction.
Sol: (c) The concept of circular paths of fixed energy was proposed by Bohr and not derived from Rutherford’s scattering experiment.
Q2. Which of the following options does not represent ground state electronic configuration of an atom?

Sol: (b) Correct configuration in ground state should be 1 s 2 2s 2 2p 6 3s 2 3p b 3d 10 4s 1
Q3. The probability density plots of Is and 2s orbitals are given in the following figures.
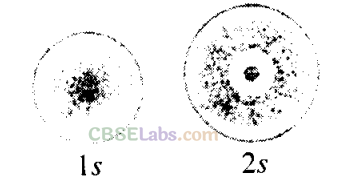
The density of dots in a region represents the probability density of finding electrons in the region.On the basis of the above diagram, which of the following statements is incorrect?
(a) 1s and 2s orbitals are spherical in shape.
(b) The probability of finding the electron is maximum near the nucleus.
(c) The probability of finding the electron at a given distance is equal in all directions.
(d) The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases.
Sol:
(d) The probability density of electrons for 2s orbital first increases then decreases and after that it begins to increase again.
Q4. Which of the following statements is not correct about the characteristics of cathode rays?
(a) They start from the cathode and move towards the anode.
(b) They travel in straight line in the absence of an external electrical or magnetic field.
(c) Characteristics of cathode rays do not depend upon the material of electrodes in cathode ray tube.
(d) Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube.
Sol:
(d) Cathode rays consist of negatively charged material particles called electron. They were discovered by William Crookes. The characteristics of cathode rays do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.
Q5. Which of the following statements about the electron is incorrect?
(a) It is a negatively charged particle.
(b) The mass of electron is equal to the mass of
neutron.
(c) It is a basic constituent of all atoms.
(d) It is a constituent of cathode rays.
Sol:
(b) The mass of electron is very small as compared to the mass of the neutron. Mass of electron = 9.1 x 10
-31
kg Mass of neutron = 1.674 x 10
-27
kg
Q6. Which of the following properties of
atom
could be explained correctly by Thomson Model of
atom
?
(a) Overall neutrality of atom.
(b) Spectra of
hydrogen
atom.
(c)
Position
of electrons, protons and neutrons in
atom
.
(d) Stability of atom.
Sol:
(a) According to Thomson model of atom, the mass of the atom is assumed to be uniformly distributed over the atom. This model was able to explain the overall neutrality of the atom.
Q7. Two atoms are said to be isobars if
(a) they have same atomic number but different mass number.
(b) they have same number of electrons but different number of neutrons.
(c) they are same number of neutrons but different number of electrons.
(d) Sum of the number of protons and neutrons is same but the number of protons is different.
Sol:
(d) Isobars have different atomic number, i.e., number of protons but same mass number, i.e., sum of number of protons and neutrons.
Q8. The number of radial nodes for 3p orbital is .
(a) 3 (b) 4 (c) 2 (d) 1
Sol:
(d) Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1
Q9. Number of angular nodes for 4d orbital is __________ .
(a) 4 (b) 3 (c) 2 (d) 1
Sol:
(c) Number of angular nodes in 4d orbital = l = 2
Q10. Which of the following is responsible
to rule
out the existence of definite paths or trajectories of electrons?
(a) Pauli’s exclusion principle
(b) Heisenberg’s uncertainty principle
(c) Hund’s rule of maximum multiplicity
(d) Aufbau principle
Sol:
(b) According to Heisenberg’s uncertainty principle, the position and velocity of an electron cannot be determined simultaneously with accuracy which rules out the existence of fixed paths.
Q11. Total number of orbitals associated with third shell will be _______.
(a) 2
(b) 4
(c) 9
(d) 3
Sol:
(c) No of orbitals in 3
rd
shell (n = 3) = n
2
= 3
2
= 9.
Q12. Orbital angular momentum depends on
(a) l
(b) n and l
(c) n and m
(d) m and s
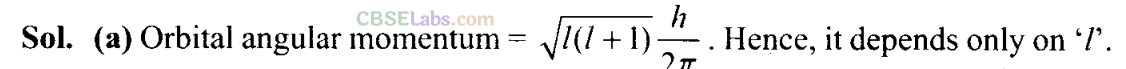
Q13. Chlorine exists in two isotopic forms, Cl-37 and Cl-35, but its atomic mass is 35.5. This indicates the ratio of Cl-37 and Cl-35 is approximately
(a) 1 : 2
(b) 1 : 1
(c) 1:3
(d) 3:1
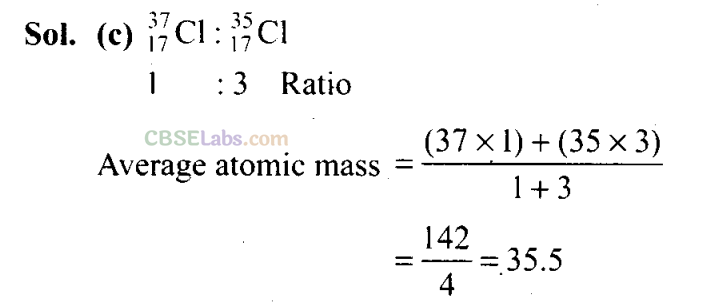
Q14. The pair of ions having same electronic configuration is _______
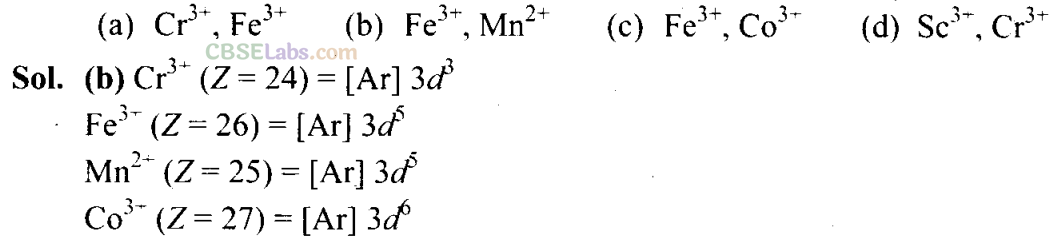

Q15. For the electrons of oxygen atom, which of the following statements is correct?
(a) Z
eff
for an electron in a 2s orbital is the same as Z
eff
for an electron in a 2p
(b) An electron in the 2s orbital has the same energy as an electron in the 2p
(c) Z
eff
for an electron in Is orbital is the same as Z
eff
for an electron in a 2s orbital.
(d) The two electrons present in the ?s orbital have spin quantum numbers m
s
but of opposite sign.

Q16. If traveling at same speeds, which of the following matter waves have the shortest wavelength?
(a) Electron
(b) Alpha particle (He
2-
)
(c) Neutron
(d) Proton
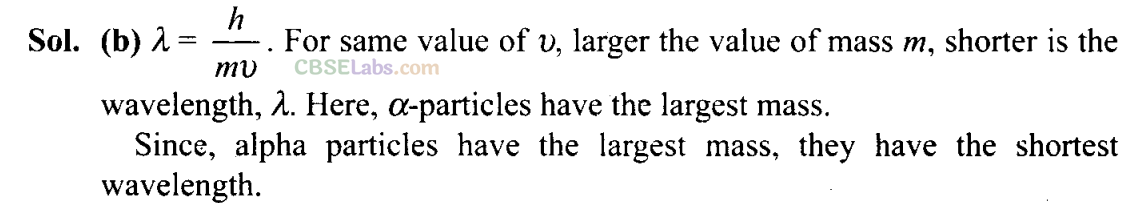
More than One Correct Answer Type
Q17. Identify the pairs which are not of isotopes?
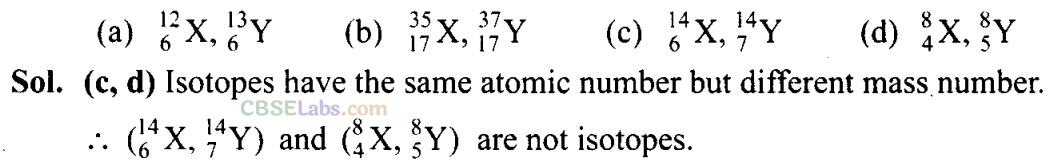
Q18. Out of the following pairs of electrons, identify the pairs of electrons present in degenerate orbitals:
(a) (i) n = 3, l = 2, m
l
= -2, m
s
= -1/2
(ii) n = 3, l= 2, m
l
= -1, m
s
= -1/2
(b) (i) n = 3, l = 1, m
l
= 1, m
s
= +1/2
(ii) n = 3, l = 2, m
l
= 1, m
s
= +1/2
(c) (i) n = 4, l = 1, m
l
= 1, m
s
= +1/2
(ii) n = 3, l = 2, m
l
= 1, m
s
= +1/2
(d) (i) n = 3, l = 2, m
l
= +2, m
s
= -1/2
(ii)n = 3, l = 2, m
l
= +2, m
s
= +1/2
Sol: (a, d) Degenerate orbitals mean the orbitals of the same sub-shell of the same main shell, i.e., their n and l values are the same. Other two pairs have different values of n and l hence, cannot be having the same energy.
Q19. Which of the following sets of quantum numbers are correct?
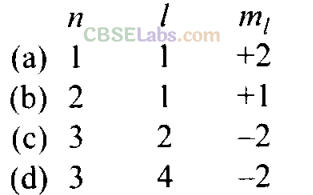
Sol:
(b, c) If n = 1, l ≠ 1. Hence, (a) is wrong.
If n = 2, l= 0, 1. For l = 1, m = -1, 0, +1. Hence (b) is correct.
If n = 3, l= 0, 1,2. For l = 2, m = -2, -1, 0, +1, +2. Hence (c) is correct.
If n = 3, l ≠4. Hence, (d) is wrong.
Q20. In which of the following pairs, the ions are isoelectronic?
Q21. Which of the following statements concerning the quantum numbers are correct?
(a) Angular quantum number determines the three dimensional shape of the orbital.
(b) The principal quantum number determines the orientation and energy of the orbital.
(c) Magnetic quantum number determines the size of the orbital.
(d) Spin quantum number of an electron determines the orientation of the spin of electron relative to the chosen axis.
Sol:
(a, d)
(a) Azimuthal quantum number l is also known as orbital angular momentum or subsidiary quantum number. It determines three-dimensional shape of the orbital.
(b) The principal quantum number determines the size of the orbit.
(c) Magnetic quantum number determines the orientation of the electron cloud in a subshell.
(d) An electron spins around its own’ axis, much in a similar way as earth spins around its own axis while revolving around the sun. In other words, an electron has, besides charge and mass, intrinsic spin angular quantum number.
Short Answer Type Questions
Q22.
Arrange s, p and d sub-shells of a shell in the increasing order of effective nuclear charge (Z
eff
) experienced by the electron present in them
Sol: .s-orbitals shield the electrons from the nucleus more effectively than p-orbitals which in turn shield more effectively than d-orbitals. Hence, the arrangement of subshells in the increasing order of effective nuclear charge is:
d<p< s
Q23. Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
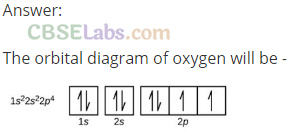
Q24. Show the distribution of electrons in oxygen atom (atomic number 8) using orbital diagram.
Sol:
Ni (28): ls
2
2s
2
2p
6
Is
2
3p
6
3d
8
4s
2
Ni
2+
(26): Is
2
2s
2
2p
6
3s
2
3p
6
3d
8
Hence, to form Ni
2+
ion, it will lose 2 electrons from 4s orbital.
Q25. Which of the following orbitals are degenerate?
3d
xy
, 4d
xv
, 3d
yz
, 3d
z
2
:
, 4d
yz
., 4dz
2
:
Sol:
Degenerate orbitals are the orbitals of the same subshell of the same main shell. Hence, these are
(3d
xy
, 3d
z
2
, 3d
yz
) and (4d
xy
, 4d
xz
, 4d
z
2
).
Q26. Calculate the total number of angular nodes and radial nodes present in 3p orbital.
Sol:
For 3p orbital, n = 3,l= 1
Number of angular nodes = 1=1
Number of radial nodes = n — l —1 = 3—1 — 1 = 1
Q27. The arrangement of orbitals on the basis of energy is based upon their (n +l) value. Lower the value of (n + l), lower is the energy. For orbitals having the same values of (n + I), the orbital with
lower
value of n will have lower energy.
I. Based upon the above information, arrange the following orbitals in the increasing order of energy.
(a) 1s, 2s, 3s, 2p
(b) 4s, 3s
,
3p, 4d
(c) 5p, 4d, 5d, 4f, 6s
(d) 5f, 6d , 7s , 7p
II. Based upon the above information, solve the questions given below:
(a) Which of the following orbitals has the lowest energy?
4d, 4f, 5s, 5p
(b) Which of the following orbitals has the highest energy?
5p, 5d, 5f 6s, 6p
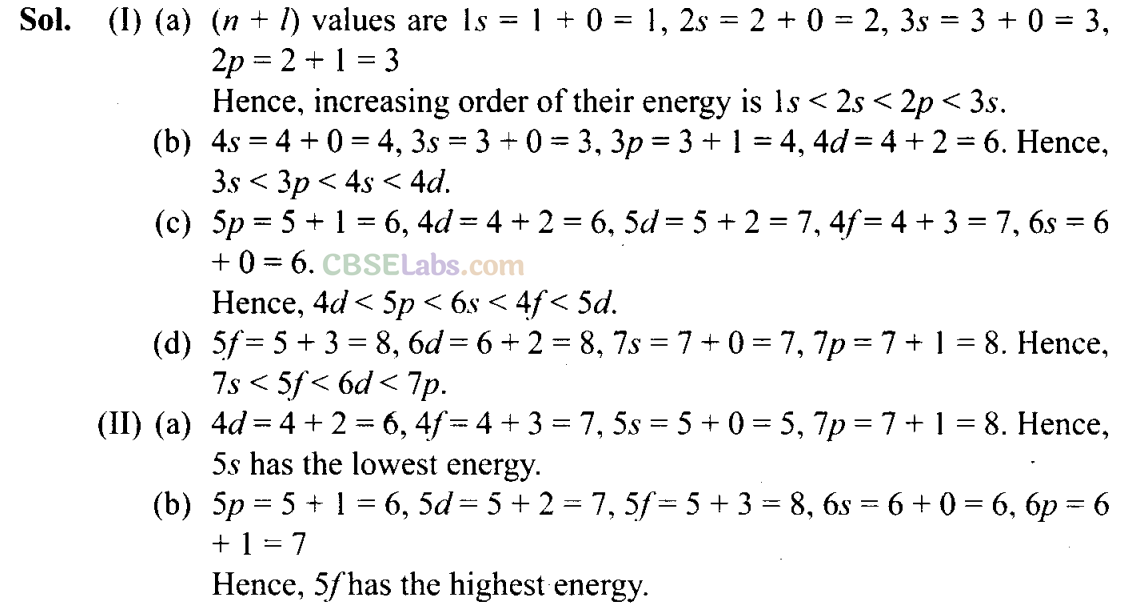
Q28. Which of the following will not show deflection from the path on passing through an electric field?
Proton, cathode rays, electron, neutron.
Sol:
Neutron, since it is neutral and cannot be deflected by an electric field.
Q29. An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
Sol:
Atomic mass number = A = 13. n = 7
As A = n + p p = A — n= 13 —7 = 6
Hence Z = p = 6
Q30. Wavelengths of different radiations are given below:
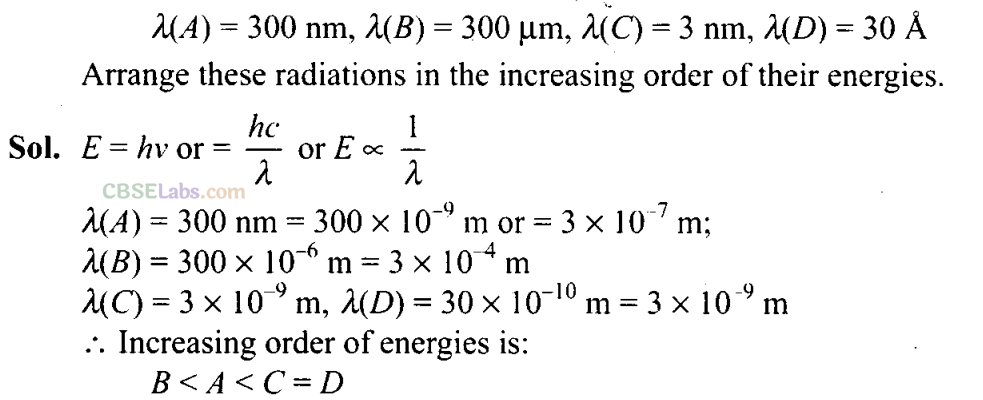
Q31. The electronic configuration of valence shell of Cu is 3d
10
4s
1
and not 3d
9
4s
2
. How is this configuration explained?
Sol:
Configuration with completely filled and half-filled orbitals have extra stability. In 3d
10
4s
1
, d-orbitals are completely filled and s-orbital is half- filled. Hence, it is a more stable configuration.
Q32. The Balmer series in the hydrogen spectrum corresponds to the transition from n
1
= 2 to n
2
= 3, 4,……… This series lies in the visible region. Calculate the wave number of line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (R
H
= 109677 cm
-1
).
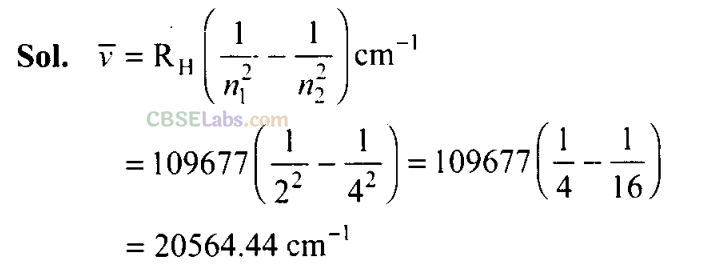
Q33. According to de Broglie, matter should exhibit dual behaviour, that is both particle and wave like properties. However, a cricket ball of mass 100 g does not move like a wave when it is thrown by a bowler at a speed of 100 km/h. Calculate the wavelength of the ball and explain why it does not show wave nature.
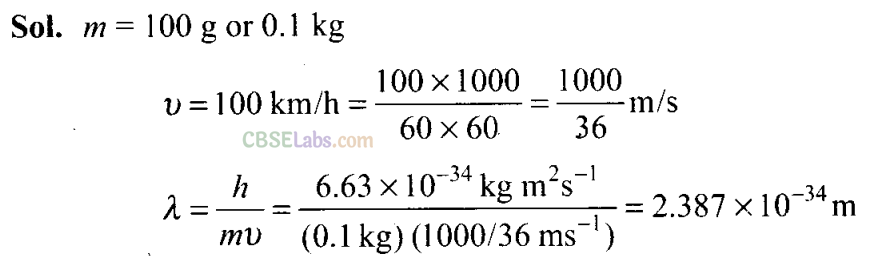
Since the wavelength is very small, the wave nature cannot be detected.
NCERT Exemplar Class 11 Chemistry Solutions
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
- Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 States of Matter
- Chapter 6 Thermodynamics
- Chapter 7 Equilibrium
- Chapter 8 Redox Reactions
- Chapter 9 Hydrogen
- Chapter 10 The s-Block Elements
- Chapter 11 The p-Block Elements
- Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
- Chapter 13 Hydrocarbons
- Chapter 14 Environmental Chemistry