Frank ICSE Class 10 Physics Solutions Heat Calorimetry
Formulae Handbook For ICSE Class 9 and 10 Educational Loans in India
Frank ICSE Class 10 Physics Solutions Heat Calorimetry
PAGE NO-247:
Solution 1
Thermal energy is energy that is powered by a heat source. For e.g.: an electric heater generates thermal energy that can be used to warm a cold room in the winter.
Solution 2
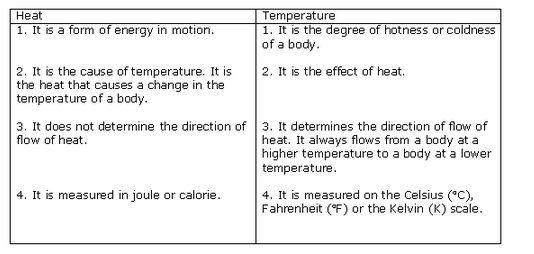
Yes, heat is a form of energy.
Solution 3
Temperature is a physical property that quantitatively expresses the common notions of hot and cold. It is the degree of hotness or coldness of a body or environment.
Solution 4
Solution 5
The SI unit of heat energy is joule (J).
Solution 6
1 joule is the amount of heat required to raise the temperature of 1 kg of a substance, that has specific heat capacity 1J/kgK, through 1
o
C.
Solution 7
1 J = 4.2 cal. So, 1 joule is bigger than 1 calorie.
Solution 8
A thermometer is used to measure temperature.
Solution 9
Solution 10
Temperature is the physical quantity that measures the degree of hotness.
Solution 11
Its energy increases on heating.
Solution 12
Gas molecules have very weak or no bonds at all and the spaces between gas molecules are very large. So, the molecules of a gas move about freely.
Solution 13
Two scales for measuring temperature arei. Celsius scaleii. Fahrenheit scale
Solution 14
‘Liquid-in-glass’ kind of thermometer is commonly used.
Solution 15
Doctor’s thermometer is also called Clinical thermometer.
Solution 16
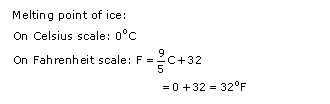
Solution 17
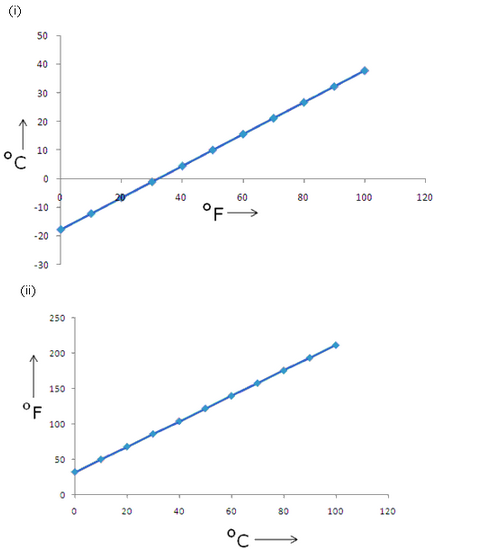
Celsius scale and Fahrenheit scale are two commonly used scales of temperature because the former is based on the freezing point of water as 0
o
C and boiling point of water as 100
o
C. The same points on the Fahrenheit scale are 32
o
F and 212
o
F.
Solution 18
The normal body temperature of a healthy person is 37
o
C.
Solution 19
![]()
Solution 20
Lower fixed point = 32
o
F Upper fixed point = 212
o
F
Solution 21
In Celsius scale, melting point of ice and boiling point of water are referred as “lower fixed point” and “upper fixed point” respectively. The temperature difference between the reference points is divided into 100 divisions and each division is called “one degree Celsius” (1
o
C). Thus, the melting point of ice is taken as 0
o
C and the boiling point as 100
o
C.
Solution 22

Solution 23
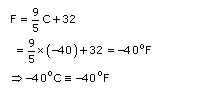
Solution 24
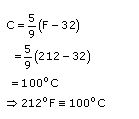
PAGE NO-248:
Solution 25
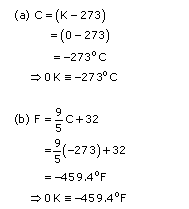
Solution 26
Absolute zero is the temperature at which volume or pressure of an ideal gas becomes nil. It is 0 degrees on the Kelvin scale, which translates to -273
o
C (or -459.4
o
F).
Solution 27

Solution 28

Solution 29
SI unit of:i. Amount of heat ? jouleii. Heat Capacity ? joule per Kelviniii. Specific Heat Capacity ? joule per kilogram per Kelvin
Solution 30

Solution 31
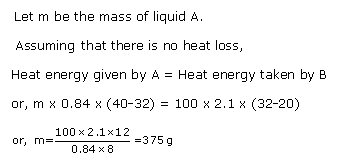
Solution 32
Specific heat capacity of water is 4200 Jkg
-1
K
-1
.
Solution 33
This means that 4200 J of heat is required to raise the temperature of 1kg of water by 1K.
Solution 34
(i) In cooling ? Water is used in the cooling systems of automobiles and other engines. (ii) As heat reservoir – In cold countries, water is used as a reservoir for wine and juice to avoid their freezing. The reason is that water can provide more heat to the bottles due to its high specific heat capacity. Hence, they do not cool down further to freeze.
Solution 35
A calorimeter is a device used to measure the quantity of heat transferred to or from an object. It is made of copper because:i. Copper is a good conductor of heat so it attains the temperature of its contents in a very short time.ii. It has low specific heat (390 Jkg
-1
K
-1
). Therefore, it will take only a very little part of the heat energy given out in the experiment.
Solution 36
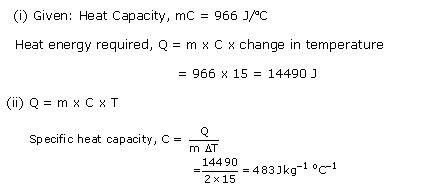
Solution 37
Farmers fill their fields with water on a cold winter night to protect the crops from frost. In the absence of water, if on a cold night the temperature of the surroundings fall below 0
0
C, then the veins of the plants shall freeze. Due to anomalous expansion of water, ice shall occupy more volume than water. As a result of this expansion, veins shall burst and crops shall be destroyed. But water sprinkled on the crops shall not allow the temperature of the veins to fall below 0
0
C.
Solution 38

Solution 39
Heat capacity of a body is the quantity of heat required to raise its temperature by 1
o
C. It depends upon the mass and the nature of the body.Units: J/
o
C or calorie/
o
C
Solution 40
Specific heat capacity is the amount of heat required to raise the temperature of 1 kg of the substance by 1
o
C. Units: j/kgK or calorie/g
o
C
Solution 41
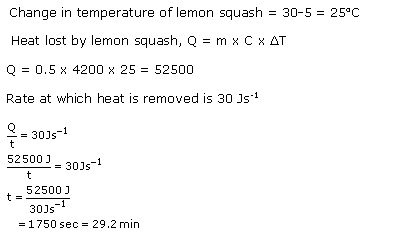
Solution 42

Solution 43
The specific heat capacity of water (4200 J Kg
-1
K
-1
) is about five times as that of sand. Due to which water takes long time to get heated up and equally long time to get cooled. Thus, large temperature difference between the land and the sea causes formation of land and sea breezes.
Solution 44
Principle of Calorimetry: When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such thatTotal heat gained by colder body = Total heat lost by the hot body,if there is no loss of heat to the surroundings.
Solution 45
Water is used as an effective coolant since it has a high value of specific heat capacity (4200 J kg
-1
K
-1
).
Physics Chemistry Biology Maths
RD Sharma Class 10 Solutions
Video Solutions