Equilibrium Class 11 Notes Chemistry Chapter 7
• Chemical Equilibrium
In a chemical reaction chemical equilibrium is defined as the state at which there is no further change in concentration of reactants and products.
For example,
![]()
At equilibrium the rate of forward reaction is equal to the rate of backward reaction. Equilibrium
mixture:
The mixture of reactants and products in the equilibrium state is called an equilibrium mixtures.
Based on the extent to which the reactions proceed to reach the state of equilibrium, these may be classified in three groups:
(i) The reactions which proceed almost to completion and the concentrations of the reactants left are negligible.
(ii) The reactions in which most of the reactants remains unchanged, i.e. only small amounts of products are formed.
(iii) The reactions in which the concentrations of both the reactants and products are comparable when the system is in equilibrium.
• Equilibrium in Physical Processes
(i) Solid-Liquid Equilibrium: The equilibrium is represented as
![]()
Rate of melting of ice = Rate of freezing of water.
The system here is in dynamic equilibriums and following can be inferred.
(a) Both the opposing processes occur simultaneously
(b) Both the processes occur at the same rate so that the amount of ice and water – remains constant.
(ii) Liquid-Vapour Equilibrium
The equilibrium can be represented as
Rate of evaporation = Rate of condensation
When there is an equilibrium between liquid and vapours, it is called liquid-vapour equilibrium.
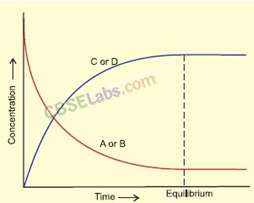
(iii) Solid-Vapour Equilibrium
This type of equilibrium is attained where solids sublime to vapour phase. For example, when solid iodine is placed in a closed vessel, violet vapours start appearing in the vessel whose intensity increases with time and ultimately, it becomes constant.
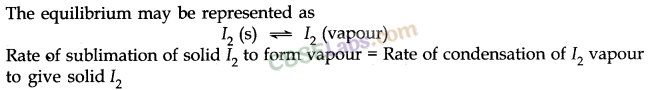
• Equilibrium involving Dissolution of Solid in Liquid
Solution:
When a limited amount of salt or sugar or any solute dissolves in a given amount of water solution is formed.
At a given temperature state is reached when no more solute can be dissolved then the solution is called saturated solution.
The equilibrium between a solid and its solution is indicated by the saturated solution and may be represented as
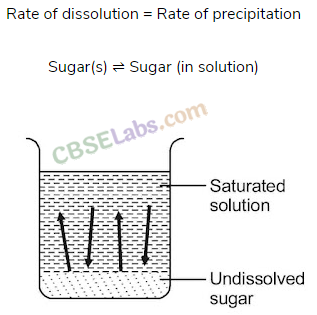
Here dissolution and precipitation takes place with the same speed.
On adding a small amount of radioactive sugar to the saturated solution it will be found that the sugar present in the solution as well as in the solid state is radioactive.
• Equilibrium between a Gas and its Solution in Liquid
This type of equilibrium can be seen by the following example:
Let us consider a sealed soda water bottle in which C02 gas is dissolved under high pressure. A state of equilibrium is attained between CO2 present in the solution and vapours of the gas.
![]()
Henry’s law:
The solubility of a gas in a liquid at a certain temperature is governed by Henry’s law. It states that the mass of a gas that dissolves in a given mass of a solvent at any temperature is proportional to the pressure of the gas above the surface of the solvent.
![]()
• Characteristics of Equilibria Involving Physical Processes
(i) The equilibrium can be attained only in closed systems at a given temperature.
(ii) At the equilibrium the measurable properties of the system remain constant.
(iii) The equilibrium is dynamic since both the forward and backward processes occur at same rate.
(iv) At equilibrium, the concentrations of substances become constant at constant temperature.
(v) The value of equilibrium constant represents the extent to which the process proceeds before equilibrium is achieved.
• Equilibrium in Chemical Processes
Like equilibria in physical systems it can also be achieved in chemical process involving reversible chemical reactions carried in closed container.
![]()
The dynamic nature of chemical equilibrium can be demonstrated in the synthesis of ammonia by Haber’s process. Haber started his experiment with the known amounts of N2 and H2 at high temperature and pressure. At regular intervals of time he determined the amount of ammonia present. He also found out concentration of unreacted N2 and H2.
After a certain time he found that the composition of mixture remains the same even though some of the reactants are still present. This constancy indicates the attainment of equilibrium. In general, for a reversible reaction the chemical equilibria can be shown by
After a certain time the two reactions occur at the same rate and the system reaches a state of equilibrium. This can be shown by the given figure.

• Equilibrium in Homogeneous System
When in a system involving reversible reaction, reactants and products are in the same phase, then the system is called as homogeneous system.
For Example,
![]()
After some time it can be observed that an equilibrium is formed. The equilibrium can be seen by constancy in the colour of the reaction mixture.
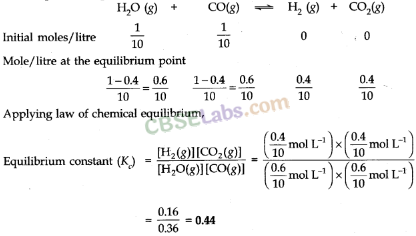
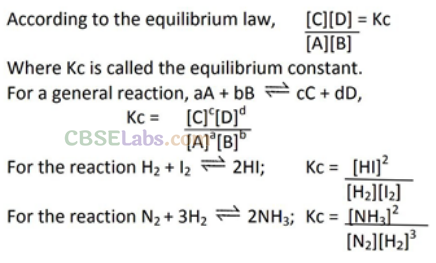
• Law of Chemical Equilibrium
At a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation. Let us consider the reaction,
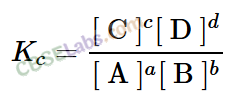
• Relationship between Equilibrium constant K, reaction Quotient Q and Gibbs energy G.
A mathematical expression of thermodynamic view of equilibrium can be described by tine equation.

• Factors Affecting Equilibria
Le Chatelier’s principle: If a system under equilibrium is subjected to a change in temperature, pressure or concentration, then the equilibrium shifts in such a manner as to reduce or to counteract the effect of change.
Effect of Change of Concentration:
When the concentration of any of the reactants or products in a reaction at equilibrium is changed, the composition of the equilibrium changes so as to minimise the effect.
Effect of Pressure Change
If the number of moles of gaseous reactants and products are equal, there is no effect of pressure.
When the total number of moles of gaseous reactants and total number of moles of gaseous products are different.
On increasing pressure, total number of moles per unit volume increases, thus the equilibrium will shift in direction in which number of moles per unit volume will be less.
If the total number of moles of products are more than the total number of moles of reactants, low pressure will favour forward reaction.
If total number of moles of reactants are more than total number of moles of products, high pressure is favourable to forward reaction.
Effect of Inert Gas Addition
If the volume is kept constant there is no effect on equilibrium after the addition of an inert gas.
Reason:
This is because the addition of an inert gas at constant volume does not change the partial pressure or the molar concentration.
The reaction quotient changes only if the added gas is involved in the reaction.
Effect of Temperature Change
When the temperature of the system is changed (increased or decreased), the equilibrium shifts in opposite direction in order to neutralize the effect of change. In exothermic reaction low temperature favours forward reaction e.g.,

but practically very low temperature slows down the reaction and thus a catalyst is used. In case of endothermic reaction, the increase in temperature will shift the equilibrium in the direction of the endothermic reaction.
Effect of a Catalyst
Catalyst has no effect on the equilibrium composition of a reaction mixture.
Reason: Since catalyst increases the speed of both the forward and backward reactions to the same extent in a reversible reaction.
• Ionic Equilibrium in Solution
Electrolytes:
Substances which conduct electricity in their aqueous solution.
Strong Electrolytes:
Those electrolytes which on dissolution in water are ionized almost completely are called strong electrolytes.
Weak electrolyte:
Those electrolytes which on dissolution in water partially dissociated are called weak electrolyte.
Ionic Equilibrium:
The equilibrium formed between ions and unionised substance is called ionic equilibrium, e.g.,
Acids:
Acids are the substances which turn blue litmus paper to red and liberate dihydrogen on reacting with some metals.
Bases:
Bases are the substances which turn red litmus paper blue. It is bitter in taste. Common Example: NaOH, Na
2
C0
3
.
• Arrhenius Concept of Acids and Bases
Acids:
According to Arrhenius theory, acids are substances that dissociates in water to give hydrogen ions H
+
(aq).
Bases:
Bases are substances that produce OH
–
(aq) after dissociation in water.
![]()
• Limitations of the Arrhenius Concept
(i) According to the Arrhenius concept, an acid gives H
+
ions in water but the H+ ions does not exist independently because of its very small size (~H
-18
m radius) and intense electric field.
(ii) It does not account for the basicity of substances like, ammonia which does not possess a hydroxyl group.
• The Bronsted-Lowry Acids and Bases
According to Bronsted-Lowry, an acid is a substance which is capable of donating a hydrogen ion H
+
and bases are substances capable of accepting a hydrogen ion H
+
.
In other words, acids are proton donors and bases are proton acceptors. This can be explained by the following example.
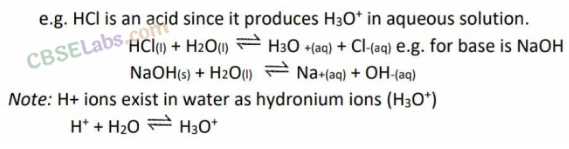
• Acid and Base as Conjugate Pairs
The acid-base pair that differs only by one proton is called a conjugate acid-base pair.
Let us consider the example of ionization of HCl in water.
![]()
Here water acts as a base because it accepts the proton.
CL is a conjugate base of HCl and HCl is the conjugate acid of base CL. Similarly, H
2
0 is conjugate base of an acid H
3
0
+
and H
3
0
+
is a conjugate acid of base H
2
O.
• Lewis Acids and Bases
According to Lewis, acid is a substance which accepts electron pair and base is a substance with donates an electron pair.
Electron deficient species like AlCl
3
, BH
3
, H
+
etc. can act as Lewis acids while species like H
2
0, NH
3
etc. can donate a pair of electrons, can act as Lewis bases.
• Ionization of Acids and Bases
Strength of acid or base is determined with the help of extent of ionization in aqueous solution.
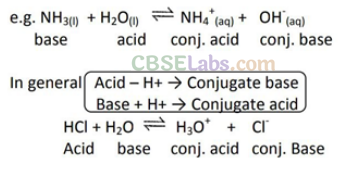
pH Scale:
Hydrogen-ion concentration are measured as the number of gram ions of hydrogen ions present per litre of solution. Since these concentrations are usually small, the concentration is generally expressed as the pH of the solution. pH being the logarithm of the reciprocal of the hydrogen ion concentration.
• Di and Polybasic Acids
Acids which contain more than one ionizable proton per molecule are called Dibasic acids or polybasic acids or polyprotic acids.
Common examples are oxalic acid, sulphuric acid, phosphoric acid etc.
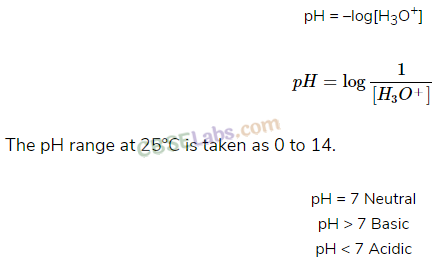
Factors Affecting Acid Strength
When the strength of H-A bond decreases

The energy required to break the bond decreases, H-A becomes a stronger acid.
As the size of A increases down the group, H-A bond strength decreases and so the acid strength increases.
In a period, as the electronegativity of A increases, the strength of the acid increases.
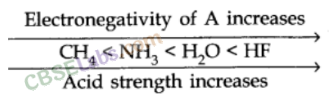
• Common Ion Effect
If in a aqueous solution of a weak electrolyte, a strong electrolyte is added having an ion common with the weak electrolyte, then the dissociation of the weak electrolyte is decreased or suppressed. The effect by which the dissociation of weak electrolyte is suppresed is known as common ion effect.
![]()
• Hydrolysis of Salts and the pH of their Solutions

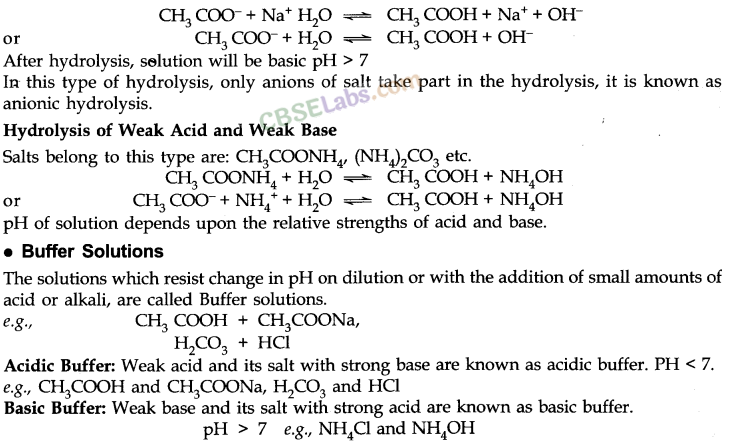
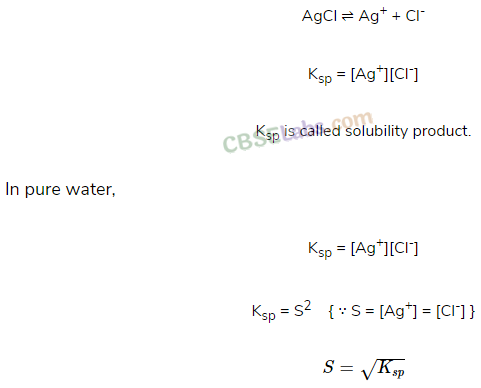
• Solubility Products
It is applicable to sparingly soluble salt. There is equilibrium between ions and unionised solid substance.
• Equilibrium:
It can be established for both physical and chemical processes. At the state of equilibrium rate of forward and backward reactions are equal.
• Equilibrium constant:
Kc is expressed as the concentration of products divided by reactants each term raised to the stoichiometric coefficients. For reactions,
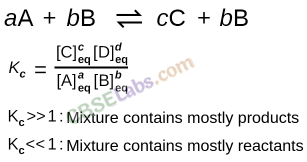
• Le Chatelier’s principle:
It states that the change in any factor such as temperature, pressure, concentration etc., will cause the equilibrium to shift in such a direction so as to reduce the effect of the change.
• Electrolytes:
Substances that conduct electricity in aqueous solutions are called electrolytes.
• Arrhenius Concept:
According to Arrhenius, acids give hydrogeneous while bases produce hydroxyl ions in their aqueous solution.
• Bronsted-Lowry concept:
Bronsted-Lowry defined acid as proton donor and a base as a proton acceptor.
• Conjugate base and Conjugate acid:
When a Bronsted-Lowry acid reacts with a base it produces its conjugate base and conjugate acid.
• Conjugate pair of acid and base:
Conjugate pair of acid and base differs only by one proton.
• Lewis acids:
Define acid as an electron pair acceptor and a base as an electron pair donor.
• pH Scale:
Hydronium ion concentration in molarity is more conveniently expressed on a logarithmic scale known as the pH scale. The pH of pure water is 7.
• Buffer solution:
It is the solution whose pH does not change by addition of small amount of strong acid or base.
For example: CH
3
COOH + CH
3
COONa.
• Solubility product (K
sp
):
For a sparingly soluble salt, it is defined as the product of molar concentration of the ions raised to the power equal to the number of times each ion occurs in the equation for solubilities.
