Electrochemistry Class 12 Notes Chemistry Chapter 3
1. Electrochemistry
is the branch of chemistry which deals with the relationship between electrical energy and chemical energy and inter-conversion of one form into another.
2. An electrochemical cell
consists of two metallic electrodes dipped in electrolytic solutions. The cells are of two types:
(a) Electrolytic cells (b) Galvanic cells
3. A galvanic cell
consists of two half cells. Each half cell contains an electrolytic solution and a metallic electrode.The electrode at which- oxidation takes place is called an anode and the electrode at which reduction takes place is called the cathode. The half-cells are separated from each other by means of a porous pot or a salt bridge.
4. The passage of current from one electrode
to the other indicates the existence of potential difference between them. This difference of potential which causes current to flow from the electrode of higher negative potential is called the electromotive force (emf).
5. Electrical energy
= Emf (volts) x Quantity of electricity (coulombs)
6. The potential of SHE
is assigned an arbitrary value of zero. E° = 0 V. It is used as a reference electrode for measuring the standard electrode potentials. .
7. When the elements
are arranged in order of their standard electrode potentials, a series known as electrochemical series is obtained.
8. Standard emf of a cell,
E
0
cell
=E
0
cathode
– E
0
node
= E
0
Riglit
– E
0
Left
9. ΔG° = -nFE
0
cell
If E
0
cell
is positive, ΔG° would be negative and reaction would be spontaneous. If E
0
cell
is
negative, ΔG° would be positive and the reaction would be non-spontaneous.
10. A species with higher standard
reduction potential has greater tendency to accept electrons to undergo reduction or vice versa.
11. The potential of an electrode
in contact with its ions in solution varies with the concentration of the ion. Thus, for a redox reaction,
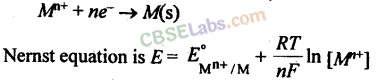
12.
For an electrochemical cell for which the overall reaction is
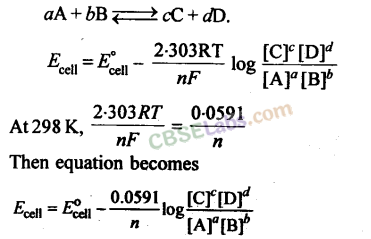
13.
The equilibrium constant, Aofcell can be relate to standard emf of cell
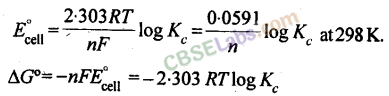
14. Resistance
is the measure of obstruction to the flow of current.
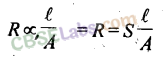
where S = specific resistance or resistivity. Unit of R is ohm.
15. Resistivity
is defined as the resistance of a conductor of 1cm length and having an area of cross-section equal to 1cm
2
.
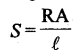
unit of resistivity is ohm-cm
16. Conductance
is reciprocal of resistance, i. e.,
Unit of conductance is ohm
-1
or mho or Siemen(S).
17. Specific conductance
Λ
sp
is reciprocal of specific resistance.
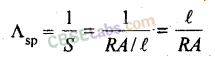
Specific conductance is thus defined as the conductance of a solution taken in a cell whose electrodes are at unit distance apart from each other and each having an area equal to 1 cm
2
. Unit of specific conductance is ohm
-1
cm
-1
or S cm
-1
.
18. Molar conductance
(Λ
m
) is defined as the conductance of the volume of solution which contains one mole of the solute and is placed between two parallel electrodes which are one centimetre apart and having sufficient area to hold the whole of the solution.
Unit of molar conductance is Ω
-1
cm
2
mol
-1
or S cm
2
mol
-1
19.
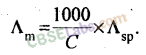
where C = concentration of solution in moles per litre (or Molarity).
20.
The electrical conductance through metals decreases with increase in temperature.
21.
The electrolytic conductance increases with increase of temperature.
22. Effect of Dilution on
(а) Equivalent conductance: The value of equivalent conductance increase with dilution and attains a maximum value at infinite dilution.
(b) Specific conductance: The value of specific conductance decreases with dilution as the number of current carrying particles i.e., ions present per cm
3
of solution decreases on dilution.
(c) Molar conductance: The value of molar conductance increases with dilution and finally attains a maximum value at infinite dilution.
23. Variation of molar conductance with concentration:
(a) Strong electrolytes:
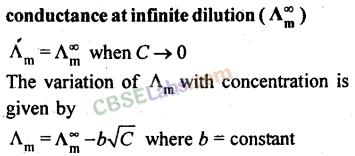
(b) Weak electrolytes:
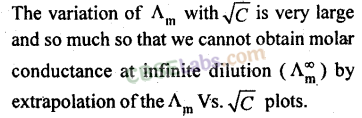
24. According to Kohlrausch law
of independent migration of ions, the limiting molar conductivity of an electrolyte can be expressed as the sum of the contributions of the cation and the anion of the electrolyte.
![]()
where v
+
, v
–
are the number of cat ions and anions per unit formula of the electrolyte respectively;
λ
0
+
, and
λ
0
–
are the limiting molar conductivities of the cation and anion respectively.
25. Faraday’s laws of electrolysis:
(a) First law:
The amount of a substance deposited or liberated at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. Mathematically,

where m = mass of substance deposited or liberated.
Mass of substance A deposited or liberated Mass of substance B deposited or liberated current in amperes t = time in seconds, and Z = constant called electrochemical equivalent.
(b) Second law:
When the same quantity of electricity is passed through solutions of different electrolytes, the weight of different substances deposited or liberated at the respective electrodes are proportional to their chemical equivalent weights.
26.

27.
The charge on one mole of electrons is approximately equal to 96500 coulombs. This quantity of electricity is called Faraday constant (F).
28. A battery
consists of two or more galvanic cells connected in series. There are two kinds of batteries:
(a) Primary batteries:
In primary batteries, when the reactants have been converted into
products, no more electricity is produced. The cell reaction cannot be reversed and the battery becomes dead.
(b) Secondary batteries:
In secondary batteries (or cells), the cell reaction can be reversed by passing electricity through the battery (charging). It means that the battery can be used again and again through a large number of discharging and charging cycles.
29.
The most common example of secondary battery is the lead storage battery.
30. Electrical cells
that are discharged to convert the energy from the combustion of fuels (hydrogen, carbon monoxide, methane, etc.) directly into the electrical energy are called fuel cells.
31. The corrosion of metals
is an electrochemical process. It occurs in presence of water and oxygen.