Students must start practicing the questions from CBSE Sample Papers for Class 12 Chemistry with Solutions Set 8 are designed as per the revised syllabus.
CBSE Sample Papers for Class 12 Chemistry Set 8 with Solutions
Time: 3 hrs
Max. Marks: 70
General Instructions
Read the following instructions carefully.
- There are 33 questions in this question paper with internal choice.
- Section A consists of 16 multiple-choice questions carrying 1 mark each.
- Section B consists of 5 short answer questions carrying 2 marks each.
- Section C consists of 7 short answer questions carrying 3 marks each.
- Section D consists of 2 case-based questions carrying 4 marks each
- Section E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
Section A
(The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.)
Question 1.
Which of the following reagent is required for the following conversion ? [1]
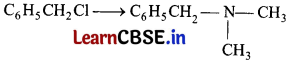
(a) CH
3
NH
2
, CH
3
Cl
(b) CH
3
Cl, RNH
2
(C) NH
3
, CH
3
Cl
(d) CH
3
CH
2
NH
2
Answer:
(a) CH
3
NH
2
, CH
3
Cl
CH
3
NH
2
and CH
3
Cl are the required reagents for the given conversion.
Question 2.
Which of the following statements is not correct for carboxylic acids? [1]
(a) Aromatic carboxylic acids undergo electrophilic substitution reactions.
(b) Carboxylic acids are stronger acids than alcohols.
(c) Carboxylic acids does not undergo any reaction with Na
2
CO
3
gas.
(d) Hell-Volhard Zelinsky reaction is given by carboxylic acids.
Answer:
(c) Carboxylic acids does not undergo any reaction with Na
2
CO
3
gas.
When carboxylic acid reacts with Na
2
CO
3
solution, carbon dioxide is evolved with a brisk effervescence along with sodium acetate.
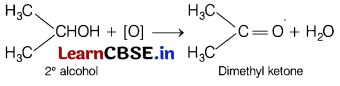
Question 3.
The major product of oxidation of secondary alcohol is [1]
(a) aldehyde
(b) ketone
(c) carboxylic acid
(d) ether
Answer:
(b) ketone
Ketones can be prepared by the oxidation of secondary alcohols by using oxidising agent such as K
2
Cr
2
O
7
/ H
2
SO
4
.
Question 4.
Arrange the following in order of increasing complexity of chemical structures. [1]
A = Fructose, B = Starch, C = Maltose
(a) A < B < C
(b) B < A <C
(c) B < C < A
(d) C < B < A
Answer:
(c) B < C < A
Fructose has molecular formula of C
6
H
12
O
6
. Maltose has molecular formula of C
12
H
22
O
11
. Starch has molecular formula of (C
6
H
10
O
5
)
n
.
Hence, the increasing order of complexity of chemical structures is
![]()
Question 5.
Which of the following alkyl halides has maximum density? [1]
(a) C
3
H
7
I
(b) C
2
H
5
I
(c) CH
3
Br
(d) CH
3
I
Answer:
(d) CH
3
I
CH 3 I has maximum density because of smallest hydrocarbon part (i.e. CH 3 ) and contain heaviest halogen (i.e. I).
![]()
Question 6.
In \(\left[\mathrm{CoF}_6\right]^{3-}\), Co
3+
uses outer d-orbitals (4d) in sp
3
d
2
-hybridisation. The number of unpaired electrons present in complex ion is [1]
(a) 0
(b) 4
(c) 2
(d) 3
Answer:
(b) 4
[CoF
6
]
3-
Here, Co is present in + 3 oxidation state.
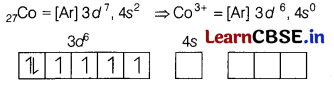
F being a weak ligand is unable to pair up its unpaired electrons thus, occupy 4s, 4p, and 4d empty orbitals as
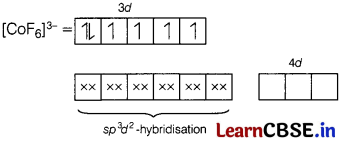
Thus, there are 4 unpaired electrons.
Question 7.
Match the properties with the metals. [1]
| (i) An element which has highest atomisation enthalpy. | (p) Mn |
| (ii) 3d-block element that show only +3 oxidation state. | (q) Zn |
| (iii) 3d-block element with lowest melting point. | (r) Sc |
| (s) Fe |
(a) (i) → (p), (ii) → (s), (iii) → (r)
(b) (i) → (s), (ii) → (q), (iii) → (r)
(c) (i) → (s), (ii) → (r), (iii) → (q)
(d) (i) → (r), (ii) → (s), (iii) → (p)
Answer:
(c) (i) → (s), (ii) → (r), (iii) → (q)
The correct match is (i)-(s), (ii)-(r), (iii)-(q)
(i) The enthalpy of atomisation is highest for iron (Fe) in 3d/-series.
(ii) Scandium (Sc) have only+3 oxidation state.
(iii) Zinc (Zn) has the lowest melting and boiling point because it does not contain any d-electrons.
Question 8.
The following diagram shows the vapour pressure curves for CH
3
F, CH
3
OH, CH
3
COOH and HCHO. [1]
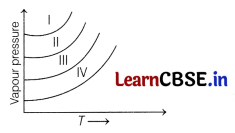
Curves I, II, III and IV respectively are for
(a) CH
3
F; HCHO; CH
3
OH; CH
3
COOH
(b) CH
3
COOH; CH
3
OH; CH
3
F; HCHO
(c) HCHO; CH
3
FCH
3
OH; CH
3
COOH
(d) CH
3
OH; CH
3
COOH; HCHO; CH
3
F
Answer:
(a) CH
3
F; HCHO; CH
3
OH; CH
3
COOH
The vapour pressure increases with decrease in intermolecular interactions. Moreover, lesser the intermolecular forces, more is the volatility and, hence higher vapour pressure at a given temperature.
Therefore, CH
3
F has highest vapour pressure, while CH
3
COOH has lowest vapour pressure.
Thus, option (a) is correct.
Question 9.
In which case, Raoult’s law is not applicable? [1]
(a) 1 M NaCl
(b) 1 M Urea
(c) 1 M Glucose
(d) 1 M Sucrose
Answer:
(a) 1 M NaCl
Raoult’s law is not applicable, if the total number of particles of solute changes in the solution due to association or dissociation. Among the given compounds, NaCl undergoes dissociation and forms Na + and Cl – ions. Therefore, Raoult’s law is not applicable to NaCl.
Question 10.
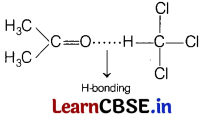
Which of the following will show a negative deviation from Raoult’s law? [1]
(a) Acetone-benzene
(b) Acetone-ethanol
(c) Benzene-methanol
(d) Acetone-chloroform
Answer:
(d) Acetone-chloroform
Acetone and chloroform will show a negative deviation due to formation of strong H-bonding after mixing and results in decrease vapour pressure.
Question 11.
The diagram below shows an incomplete experimental set-up needed to measure the E
cell
of a cell composed of the standard Cu
2+
/ Cu electrode and an Ag
+
/ Ag electrode. [1]
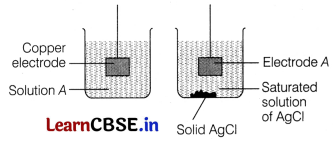
What is the chemical composition of solution A?
(a) CuSO
4
(b) AgSO
4
(c) CuCl
2
(d) AgCl
Answer:
(a) CuSO
4
Solution A = CuSO 4
![]()
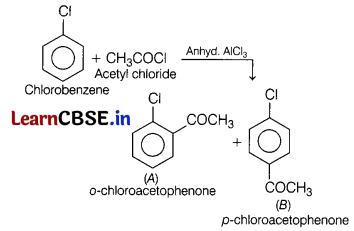
Question 12.
In the reaction, [1]

A and B are
(a) o-chloroacetophenone, methyl chloride
(b) p-chloroacetophenone, acetophenone
(c) o-chloroacetophenone, p-chloroacetophenone
(d) None of the above
Answer:
(c) o-chloroacetophenone, p-chloroacetophenone
Direction (Q. Nos. 13-16) In the following questions as Assertion (A) is followed by a corresponding Reason (R). Use the following keys to choice the appropriate answer.
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question 13.
Assertion (A) Crystal structure of oxides of transition metals often show defects.
Reason (R) Ligand field effect causes distortions in crystal structures. [1]
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
Question 14.
Assertion (A) o-nitrophenol is less soluble in water than the m-and p-isomers.
Reason (R) m-and p-nitrophenols exist as associated molecules. [1]
Answer:
(b) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
Due to the presence of intramolecular hydrogen bonding, o-nitrophenol does not form hydrogen bonds with H 2 O but m and p-nitrophenol form hydrogen bonds with water. Hence, o-nitrophenol is less soluble in water than the m and p-isomers.
Question 15.
Assertion (A) In order to convert R—Cl to pure R—NH
2
, Gabriel phthalimide synthesis can be used.
Reason (R) With proper choice of alkyl halides, phthalimide synthesis can be used to prepare 1°, 2° or 3° amines. [1]
Answer:
(c) (A) is true, but (R) is false.
(A) is true but (R) is false. It is because only primary aliphatic amines can be prepared by Gabriel-phthalimide reaction.
Question 16.
Assertion (A) Glucose does not form the hydrogen bisulphite addition product.
Reason (R) Glucose is not so reactive to form the product with NaHSO
3
. [1]
Answer:
(c) (A) is true, but (R) is false.
Glucose does not form the hydrogen bisulphite addition product because it has cyclic structure in which —CHO group is not free to react.
Therefore, (A) is true but (R) is false.
Section B
(This section contains 5 questions with internal choice in one question. The following questions are very short answer type and carry 2 marks each.)
Question 17.
A 4% solution (w/w) of sucrose (M = 342 g mol
-1
) in water has a freezing point of 271.15 K. Calculate the freezing point of 5% glucose (M = 180 g mol
-1
) in water. [2]
(Given : Freezing point of pure water = 273.15 K)
Answer:
4% solution (w/w) of sucrose is given
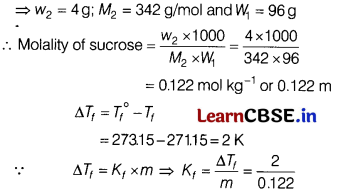
Now, molality of glucose solution
Given w
2
= 5g; M
2
= 180 g /mol; W
1
= 95g
∴ Molality of glucose = \(\frac{W_2 \times 1000}{M_2 \times W_1}\)
= \(\frac{5}{180}\) × \(\frac{1000}{95}\)
= 0.292 m
∴ ∆T
f
= K
f
× m
= \(\frac{2}{0.122}\) × 0.292 = 4.8
∴ Freezing point of glucose solution = 273.15 – 4.8
= 268.35 K
Question 18.
Explain how and why will the osmotic pressure be affected when external pressure applied becomes
(a) more than osmotic pressure.
(b) less than osmotic pressure. [2]
Answer:
(a) When applied or external pressure is more than osmotic pressure, then pure water is squeezed out by a semipermeable membrane from the solvent, then the phenomenon of reverse osmosis takes place.
(b) When external pressure is less than osmotic pressure, then the solvent starts flowing in to the solution through semipermeable membrane.
Question 19.
Account for the following. [2]
(a) Presence of a-hydrogen in aldehydes and ketones is essential for aldol condensation.
Answer:
The presence of alpha hydrogen in aldehydes and ketones is essential for aldol condensation because they are acidic in nature (due to the presence of electron withdrawing carbonyl group).
As a result, the electron density at α C— H bond decreases and hence, H-atom becomes weakly held which can be easily abstracted by strong bases forming enolate ion, which are stabilised by resonance.
(b) 3-hydroxypentan-2-one shows positive Tollen’s test.
Answer:
The structure of 3-hydroxypentan-2-one is
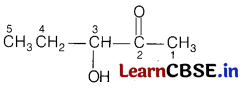
All α-hydroxy ketones gives Tollen’s test. Since, they have the ability to tautomerises to aldehydes and aldehydes gives Tollen’s test.
As the given compound is also α-hydroxy ketone and hence, it gives this test.
![]()
Question 20.
Give reason for the following. [2]
(a) Denaturation of proteins results in the loss of biological activity of the proteins.
Answer:
During the denaturation of proteins, the secondary and tertiary structures get destroyed and only the primary structure is preserved. Covalent bonds are broken and interaction between amino acid chains get disordered.
Consequently, denaturation leads to loss of biological activity of proteins.
(b) Vitamins B and C cannot be stored in our body.
Answer:
Vitamins B and C are soluble in water which must be supplied regularly in diet because they are readily excreted in urine and cannot be stored (except vitamin B
12
) in our body.
Or
(a) Name the deficiency diseases resulting from lack of vitamin A and E in the diet. [2]
Answer:
The deficiency disease due to lack of vitamin A is xerophthalmia and due to lack of vitamin E is increased fragility of RBCs and muscular weakness.
(b) Out of the four bases, name those which are common to both DNA and RNA and why DNA and RNA are called acids?
Answer:
Adenine, guanine and cytosine are present in both DNA and RNA.
DNA and RNA are considered as acids because they are formed from phosphate groups and these phosphates can readily remove a proton and make DNA and RNA highly acidic.
Question 21.
When a coordination compound CoCl
3
∙ 6NH
3
is mixed with AgNO
3
, 3 moles of AgCl are precipitated per mole of the compound. Write the
(a) structural formula of the complex.
(b) IUPAC name of the complex. [2]
Answer:
(a) When one mole of COCl
3
∙ 6H
2
O is mixed with AgNO
3
, three moles of AgCl are precipitated which indicates that three ionisable chloride ions in the complex are present.
Hence, its structural formula is [Co(NH
3
)
6
]Cl
3
.
(b) IUPAC name of the complex [Co(NH 3 ) 6 ]Cl 3 is hexaamminecobalt (III) chloride.
Section C
(This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each.)
Question 22.
An organic compound with molecular formula C
9
H
10
O, forms 2,4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzene dicarboxylic acid. Identify the compound. [3]
Answer:
- As the given compound with molecular formula C 9 H 10 O, forms a 2,4-DNP derivative and reduces Tollen’s reagent, thus it must be an aldehyde.
- As it undergoes Cannizzaro reaction, hence —CHO group is directly attached to the benzene ring.
- On vigorous oxidation, it gives 1,2-benzene dicarboxylic acid.
Therefore, it must be an ortho-substituted , benzaldehyde and the only o-substituted aromatic aldehyde which have C 9 H 10 O molecular formula is o-ethyl benzaldehyde.
Question 23.
Write the equations for the following reaction. [3]
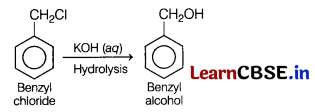
(a) Benzyl chloride is treated with aqueous KOH followed by hydrolysis.
Answer:
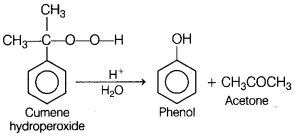
(b) Cumene hydroperoxide undergoes acid hydrolysis.
Answer:
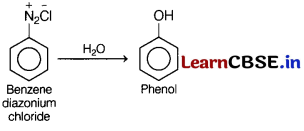
(c) Benzene diazonium chloride is treated with water.
Answer:
Question 24.
A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO
4
and ZnSO
4
until 2.8 g of Fe deposited at the cathode of cell X. How long did the current flow? Calculate the mass of Zn deposited at the cathode of cell Y.
(Molar mass : Fe = 56 g mol
-1
Zn = 65.3 g mol
-1
, 1F = 96500 C mol
-1
) [3]
Answer:
![]()
∵ 56 g of Fe is deposited by 2F or 2 × 96500 C charge
∴ 2.8 g of Fe will be deposited by
\(\frac{96500 \times 2 \times 2.8}{56}\) C charge = 9650 C
Now, Q = lt
∴ Time, t = \(\frac{9650}{2}\) = 4825s
zn
2+
+ 2e
–
→ zn
∵ Time, t = \(\frac{9650}{2}\) = 4825 s
zn
2+
+ 2e
–
→ zn
∴ 2 × 96500 C charge deposits Zn = 65.3g
∴ 9650 C charge will deposit
Zn = \(\frac{65.3 \times 9650}{96500 \times 2}\) = 3.27 g
Question 25.
For the complex [Fe(en)
2
Cl
2
] Cl, explain the
(a) type of hybridisation,
(b) magnetic behaviour and
(c) number of its geometrical isomers. [3]
Answer:
(a)
[Fe(en)
2
Cl
2
]Cl
For the given compound, Fe is in + 3 oxidation state. Hence, its electronic configuration will be Fe
3+
= 3d
5
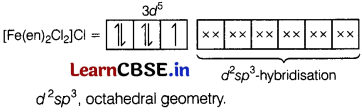
(b) Paramagnetic (as it contains one unpaired electron).
(c) Two geometrical isomers, cis and trans.
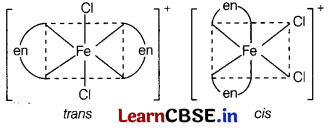
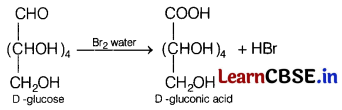
Question 26.
(a) Identify the products when D-glucose is treated with Br
2
water. [3]
(b) Why does the amino acids behave like salts rather than simple amines or carboxylic acids?
Answer:
(a)
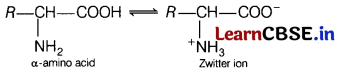
(b) In aqueous solution, the carboxyl group loses a proton and the amino group accepts a proton. In other words, the carboxyl and amino groups neutralise each other to form Zwitter ion. Due to formation of Zwitter ion, amino acids behave as salts rather than simple amines or carboxylic acids.
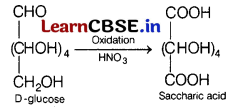
Or
(a) Name the carbohydrates which will yield saccharic acid on their reaction with HNO
3
. Write the reaction involved.
(b) Glucose and fructose are reducing sugars. Why? [3]
Answer:
(a)
It is saccharic acid which is formed as per the reaction given below.
(b) Glucose and fructose are reducing sugars because they both contain a free aldehydic and ketonic group which makes them undergo oxidation readily to form carboxylic acid and in the process, the reactive reagents are reduced easily.
![]()
Question 27.
Answer the following questions. [3]
(a) A first order reaction takes 40 min for 30% decomposition. Calculate the rate constant for this reaction. (Given, log 1.428 = 0.1548)
Answer:
To find t
1/2
, first calculate k by using the formula,
k = \(\frac{2.303}{t}\)log\(\frac{[R]_0}{[R]}\)
For a reaction,
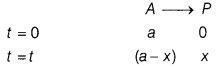
For 30% decomposition, it takes 40 min which means after 40 min. reactant reft is 70% of its initial concentration.
(b) Calculate t
1/2
for this reaction.
Answer:
Half-life of the reactant(t
1/2
)
= \(\frac{0.693}{k}\)
= \(\frac{0.693}{0.00891}\)
= 77.75 min
(c) Explain why does the rate of a reaction not remain constant throughout the reaction?
Answer:
∵ r ∝ [concentration]
Order

Hence, as time increases rate decreases.
Question 28.
Give reason for the following observations.
(a) Chloroform is stored in dark coloured bottles. [3]
Answer:
Chloroform is stored in dark coloured bottles as in presence of light, it gets converted into highly poisonous substance, phosgene (COCl
2
)
(b) Alkyl halides though polar are immiscible in water.
Answer:
To be miscible with (or soluble in water) water, the solute-water force of attraction must be stronger than the solute-solute and water-water forces of attraction. Alkyl halides are polar molecules and so held together by dipole-dipole interactions. Similarly, strong H-bonds exist between the water molecules.
The new force of attraction between the alkyl halides and water molecules is weaker than the forces of attraction already existing between alkyl halide-alkyl halide and water-water molecules.
Hence, alkyl halides (though polar) are immiscible with (or insoluble in) water.
(c) In the pair of (CH
3
)
3
C—Cl and CH
3
Cl, CH
3
Cl will react faster in S
N
2 reaction with \(\overline{\mathrm{O}}\) H.
Answer:
In S
N
2 reactions, reactivity depends upon the steric hindrance CH
3
Cl being 1° halide will react faster as compared to (CH
3
)
3
CCl which is a 3° halide because 1° halides undergo S
N
2 mechanism faster than 3° halides.
Section D
(The following questions are case-based questions. Each question has an internal choice and carries 4(1+1+2) marks each. Read the passage carefully and answer the questions that follow.)
Question 29.
Amines constitute one of the most important class of organic compounds. Amines are alkyl or aryl derivatives of ammonia formed by replacement of one or more hydrogen atoms. Alkyl derivatives are called aliphatic amines and aryl derivatives are known as aromatic amines. Both aliphatic and aromatic primary amines can be prepared by the reduction of nitro compounds catalytically with H
2
in the presence of active metal in acidic medium.
The presence of aromatic amines can be identified by performing dye test. Aniline is the simplest example of aromatic amine. It undergoes electrophilic substitution reactions in which —NH 2 group strongly activates the aromatic ring through delocalisation of lone pair of electrons of N-atom.
Aniline undergoes electrophilic substitution reactions. Ortho and para-positions to the —NH 2 group become centres of high electron density.
Thus, —NH 2 group is ortho and para-directing powerful activating group.
Answer the following questions. [4]
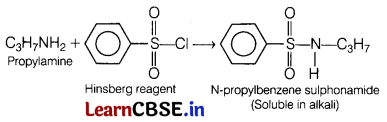
(a) Propylamine reacts with which reagent to form an alkali soluble product? (1)
Answer:
Propylamine reacts with Hinsberg reagent to form an alkali soluble product, i.e. N-propylbenzene
sulphonamide.
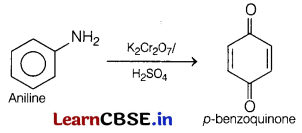
(b) Which compound is produced by oxidation of aniline with K
2
Cr
2
O
7
/H
2
SO
4
?
Answer:
The compound formed as a product is p-benzoquinone, when oxidation of aniline is carried out with K
2
Cr
2
O
7
in presence of H
2
SO
4
.
The reaction involved is as follows
(c) Convert ethyl iodide to ethyl amine. Name all the compound involve in various steps.
Answer:
The series of reaction that takes place during the conversion of ethyliodide to ethylamine is as follows
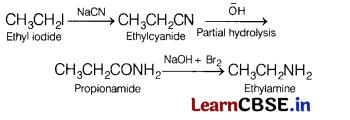
Or
Suggest a route by which the following conversion can be accomplished. Also with the name of all the compound involve in the steps. [4]
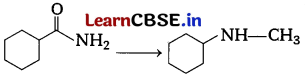
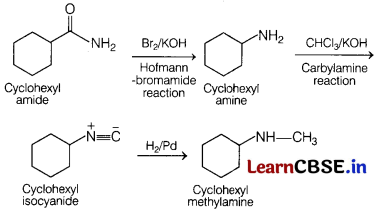
Answer:
Complete conversion can be performed as
Question 30.
For any chemical reaction, thermodynamics tells about the feasibility of a reaction chemical equilibrium tells about the extent to which a reaction will proceed and chemical kinetics tells about the rate at which a chemical reaction proceeds and factors which control this rate. When one or more substances undergo a change which results in the formation of a new products, it is called chemical reaction order and molecularity are the properties of a reaction, which helps in understanding its mechanism.
Answer the following questions. [4]
(a) For the reaction A → B, then rate of reaction becomes three times when the concentration of A is increased by nine time. What is the order of reaction?
Answer:
r
1
= k[A]
x
……. (i)
r
2
= k[9A]
x
….. (ii)
On dividing Eq. (ii) by Eq. (i), we get
\(\frac{r_2}{r_1}\) = \(\frac{k[9 A]^x}{k[A]^x}\) ⇒ 3 = (9)
x
⇒ (3)
1
= (3
2
)x
⇒ 2x = 1 ⇒ x = 1/2
Thus, the order of reaction is 1/2.
Or
For a reaction A + B → P, the rate law is given by, r = k[A]
2
[B]
2
. What is the order of this reaction? [4]
Answer:
For a reaction, A + B → P
Given, rate of a reaction, r = k [A]
1/2
[B]
2
Order w.r.t. A = \(\frac{1}{2}\);
Order w,r.t. B = 2
∴ Overall order of a reaction = 1/2 + 2 = 5/2
(b) Identify the order of a reaction, if the units of its rate constant are
(i) L
-1
mol
(ii) L mol
-1
s
-1
Answer:
The units of rate constant depends upon the order of reaction.
(i) Zero order L
-1
mol s
-1
(ii) Second order L mol
-1
s
-1
(c) Discuss any four factors which affect the rate of a chemical reaction.
Answer:
Factors influencing the rate of a chemical reaction are
- Nature of reactants: Different reactants require different amount of energies for breaking the old bonds and for the formation of new bonds. Hence, the reactivity of a substance is related to the ease with which the specific bonds are broken or formed.
- Concentration of reactants: Rate of reaction is directly proportional to the concentration of the reactants.
- Temperature: Rate of reaction increases with increase in temperature.
- Catalyst: It alters the rate of reaction without being consumed in the reaction. It provides an alternative path to the reaction with a low energy barrier.
Section E
(The following questions are long answer type and carry 5 marks each. All questions have an internal choice.)
Question 31.
(a) Why does the conductivity of a solution decreases with dilution? [5]
Answer:
Conductivity of a solution is related with the number of ions present per unit volume of the solution. When the solution is diluted, the number of ions per unit volume decreases. Hence, conductivity or specific conductance of the solution decreases.
(b) Write the reaction involved in the working of H
2
– O
2
fuel cell.
Answer:
The electrode reactions involved in the working of H
2
– O
2
fuel cell are as follows At cathode,
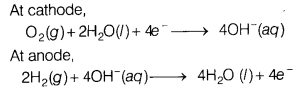
(c) What is the potential of hydrogen electrode which is in contact of a solution whose pH is 10?
Answer:
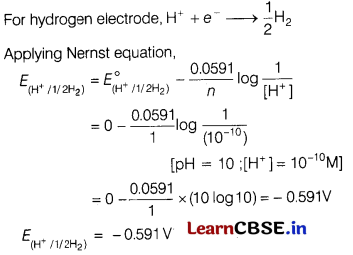
Or
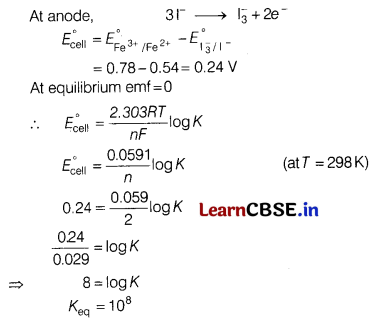
(a) Calculate the equilibrium constant for the reaction, [5]
2Fe
3+
+ 3I
–
\(\rightleftharpoons\) 2Fe
2+
+ \(\mathrm{I}_3^{-}\)
The standard reduction potential in acidic condition is 0.78 V and 0.54 V respectively, for Fe
3+
/Fe
2+
and \(\mathrm{I}_3^{-}\) /I
–
couples.
Answer:

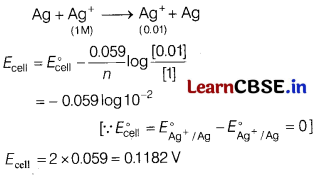
(b) For a cell,
Ag(s) | AgNO
3
(0.01 M) || AgNO
3
(1.0 M) | Ag(s)
What is the emf of the cell at 25°C?
Answer:
Electrochemical reaction is
![]()
Question 32.
Attempt any five of the following. [5]
(a) Why Cu
+
ion is not known in aqueous solution?
Answer:
In aqueous solution, copper (I) undergoes disproportionation reaction.
2Cu
+
(ag) → Cu
2+
+ Cu(s)
The highest stability of Cu
2+
ion in aqueous solution is due to negative enthalpy of hydration.
Hence, Cu
+
is not known (or unstable) in aqueous solution.
(b) Why Mn
3+
is a good oxidising agent?
Answer:
The outer electronic configuration of Mn
3+
is d
4
configuration. It has maximum tendency to gain one electron to change into stable half-filled configuration, i.e. d
5
. Therefore, it is the strongest oxidising agent.
(c) Why Ni
2+
is more stable than Pt
2+
whereas Pt
4+
is more stable than Ni
4+
?
Answer:
Ni
2+
compounds have the sum of first two ionisation enthalpies to be lower than that of Pt
2+
compounds and are thermodynamically stable.
However, the sum of first four ionisation enthalpies of Pt
4+
is lower than that of first four IEs of Ni
4+
and is relatively stable.
(d) Explain the observation, Zn, Cd and Hg are quite soft and have low melting point.
Answer:
Zn, Cd, Hg metals have completely filled d-orbitals (d
10
). It means thatd-electrons are not readily available for metallic bond formation. Since, the metallic bonds are weak.
Therefore, these metals are quite soft and also have low melting points.
(e) What is meant by ‘disproportionation reactions’?
Answer:
The disproportionation reactions are those in which the same substance gets oxidised as well as reduced.
When a particular oxidation state becomes less stable relative to other oxidation states (one lower and one higher), it undergoes disproportionation.
(f) Complete and balance the reaction.
Fe
2+
+ Mn\(\mathrm{O}_4^{-}\) + H
+
→
Answer:
5Fe
2+
+ Mn\(\mathrm{O}_4^{-}\) + 8H
+
→ Mn
2+
+ 5Fe
3+
+ 4H
2
O
(g) Why transition metals exhibit variable oxidation states?
Answer:
ns and (n – 1)d electrons of transition metal have very little difference in the energies and hence both can participate in bonding, which results in variable oxidation states.
When ns electrons take part in bonding, they exhibit lower oxidation states whereas when (n – 1)d electrons along with ns electrons participate in bonding, they exhibit variable oxidation states.
question 33.
An organic compound (A) having molecular formula C
2
H
6
O on oxidation with Na
2
Cr
2
O
7
/H
2
SO
4
produces a compound (B) which reduces Tollen’s reagent.
Both (A) and (B) produce a yellow solid on treatment with I
2
/ OH
–
. Identify A and B and write all the involved reactions. [5]
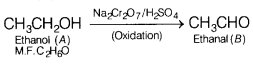
Answer:
Since, an organic compound (A) with molecular formula C
2
H
6
O on oxidation with Na
2
Cr
2
O
7
/H
2
SO
4
gives compound (S) which reduces Tollen’s reagent, therefore, (A) must be ethanol and (B) must be ethanal (S).
Since ethanol (A), CH
3
CH
2
OH contains the group
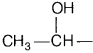
and ethanal (B), CH
3
CHO contain the group CH
3
CO—, therefore, both these on treatment with I
2
/ OH
–
undergo iodoform reaction to give yellow solid of iodoform.
Or
A hydrocarbon, P, with the formula C 6 H 12 readily decolourises bromine. [5]
(a) On reaction with hot, concentrated, acidified potassium manganate (VII) solution a single organic product, Q, is obtained. Q gives an orange precipitate when reacted with 2, 4-dinitrophenylhydrazine, 2, 4-DNP reagent, but has no reaction with Tollen’s reagent.
(i) Identify P and Q and write their IUPAC names.
(ii) Write down the reaction for the formation of Q from P.
(iii) Write down the reaction to prepare propanol from compound Q.
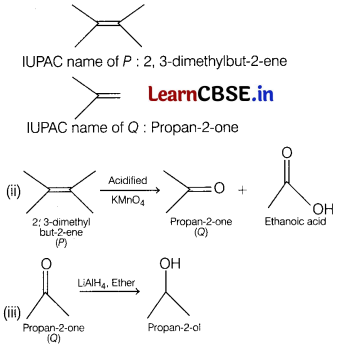
Answer:
(i) Decolourisation of bromine shows that P has a C = C double bond and is an alkene.
A single organic product on reaction with hot, concentrated acidified potassium manganate (VII) implies P is symmetrical.
Reaction with 2, 4-DNP implies 0 contains a carbonyl group.
No reaction with Tollen’s reagent implies that 0 is a ketone and not an aldehyde.
(b) Arrange the following compounds in the increasing order of their property as indicated.
(i) Acetaldehyde, acetone, di-ferf-butyl ketone, methyl fert-butyl ketone (reactivity towards HCN).
(ii) Benzoic acid, 4-nitrobenzoic acid, 3, 4-dinitrobenzoic acid, 4 methoxy benzoic acid (acid strength).
Answer:
(i) Di-tertiary butyl ketone < methyl tertiary butyl ketone < acetone < acetaldehyde.
(ii) 4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid < 3, 4-dinitrobenzoic acid.