Students must start practicing the questions from CBSE Sample Papers for Class 11 Chemistry with Solutions Set 4 are designed as per the revised syllabus.
CBSE Sample Papers for Class 11 Chemistry Set 4 with Solutions
Time Allowed : 3 hours
Maximum Marks: 70
General Instructions:
- There are 33 questions in this question paper with internal choice.
- Section – A consists of 16 multiple -choice questions carrying 1 mark each.
- Section – B consists of 5 short answer questions carrying 2 marks each.
- Section – C consists of 7 short answer questions carrying 3 marks each.
- Section – D consists of 2 case – based questions carrying 4 marks each.
- Section – E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
- Use of log tables and calculators is not allowed.
Section-A
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Question 1.
Which of the following statements are true regrading Avogrado’s Law.
(A) A given compound always contains exactly the same proportion of elements by weight.”
(B) Equal volumes of gases at the same temperature and pressure should contain equal number of molecules”.
(C) When gases combine or are produced in a chemical reaction they do so in a simple ratio by volume provided all gases are at same temperature and pressure.”
(D) “A chemical compound is always found to be made up of the same elements combined together in the fixed proportion by weight.”
Answer:
(B) Equal volumes of gases at the same temperature and pressure should contain equal number of molecules”.
Question 2.
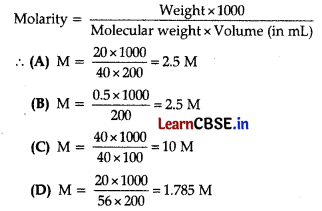
Which of the following solutions have the same concentration?
(A) 20 g of NaOH in 200 mL of solution
(B) 0.5 mol of KCl in 200 mL of solution
(C) 40 g of NaOH in 100 mL of solution
(D) 20 g of KOH in 200 mL of solution
Answer:
(A) 20 g of NaOH in 200 mL of solution
Explanation: Since,
![]()
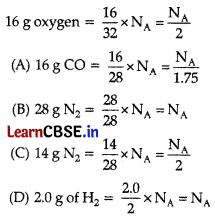
Question 3.
16 g of oxygen has same number of molecules as in:
(A) 16 g of CO
(B) 28 g of N
2
(C) 14 g of N
2
(D) 2.0 g of H
2
Answer:
(C) 14 g of N
2
Explanation:
Number of molecules Mass.
= \(\frac{\text { Mass }}{\text { Molar mass }}\) × N
A
where, NA = Avogadro number Number of molecules in
Question 4.
An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
(A) 5
(B) 7
(C) 6
(D) 9
Answer:
(C) 6
Explanation: Mass number = 13
Number of neutrons = 7
Mass number = No. of protons + No. of neutrons
13 = No. of protons + 7
No. of protons = 13-7 = 6
Atomic number = No. of protons = 6
Question 5.
The enthalpy of elements in their standard states are taken as zero. The enthalpy of formation of a compound:
(A) is always negative
(B) is always positive
(C) may be positive or negative
(D) is never negative
Answer:
(C) may be positive or negative
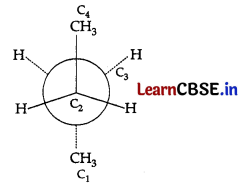
Question 6.
In the given conformation C
2
is rotated about C
2
-C
3
bond anticlockwise by an angle of 120°, then the conformation obtained is:
(A) 3-hydroxy-2-bromopropane
(B) 2-bromo-l-propanol
(C) 2-bromo-3-propanol
(D) 3-hydroxy isopropyl bromide .
Answer:
(B) 2-bromo-l-propanol
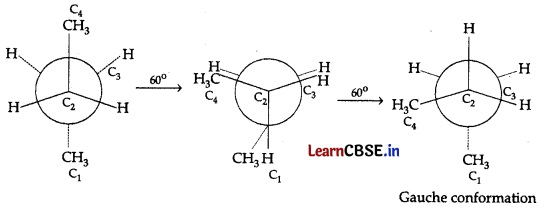
Question 7.
In the given conformation C
2
is rotated about C
2
-C
3
bond anticlockwise by an angle of 120°, then the conformation obtained is:
(A) Fully eclipsed conformation
(B) Partially eclipsed conformation
(C) Gauche conformation
(D) Staggered conformation
Answer:
(C) Gauche conformation
Explanation:
Question 8.
An alkyl halide may be converted into an alcohol by:
(A) Elimination
(B) Addition
(C) Substitution
(D) Dehydrohalogenation
Answer:
(C) Substitution
Explanation:
Alkyl halides on alkaline hydrolysis get converted into alcohols. This takes place by a nucleophilic substitution reaction where the – X atom is substituted by a nucleophile i.e – OH group.
Question 9.
The function of A1C1
3
in Friedel-Craft’s reaction is:
(A) To absorb HC1
(B) To absorb water
(C) To produce nucleophile
(D) To produce electrophile
Answer:
(D) To produce electrophile
Explanation:
The function of AlCl
3
in friedel- Crafts reaction, is to produce electrophile which later attcack on benzene.
![]()
Question 10.
In the case of homologous series of alkanes, which one of the following statements is incorrect?
(A) The members of the series are isomers of each other
(B) The members of the series have similar chemical properties
(C) The members of the series have the general formula C
n
H
2n+2
, where n is an integer
(D) The difference between any two successive members of the series corresponds to 14 unit of relative atomic mass
Answer:
(A) The members of the series are isomers of each other
Explanation:
The difference between any two successive members of the homologous series -CH
2
– i.e., the molecular weight of every two adjacent members differ by 14. (CH
2
= 12 + 2 = 14)
Question 11.
When benzene is treated with excess of Cl
2
in the presence of sunlight, the end product is:
(A) Monochlorobenzene
(B) Trichlorobenzene
(C) Hexachlorobenzene
(D) Benzene hexachloride
Answer:
(C) Hexachlorobenzene
Question 12.
A metallic carbide on treatment with water gives a colourless gas which burns readily in air and which gives a precipitate with ammoniacal silver nitrate solution. Gas evolved is:
(A) Methane
(B) Ethane
(C) Acetylene
(D) Ethylene
Answer:
(C) Acetylene
Explanation:
Gas evolved is acetylene.
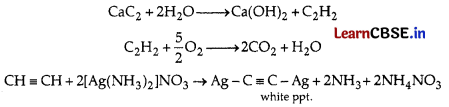
Question 13.
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): The empirical mass of Ethene is half of its molecular mass.
Reason (R): The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
Select the most appropriate answer from the options given below:
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is not the correct explanation of A.
(C) A is true but R is false.
(D) A is false but R is true.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Explanation: As molecular formula gives exact number of atoms present in the compound, so molecular formula is more informative.
![]()
Question 14.
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): Combustion of all organic compounds is an exothermic reaction.
Reason (R): The enthalpies of all elements in their standard state are zero.
Select the most appropriate answer from the options given below:
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is not the correct explanation of A.
(C) A is true but R is false.
(D) A is false but R is true.
Answer:
(B) Both A and R are true but R is not the correct explanation of A.
Explanation:
Reason (R): Combustion reactions breaks the bonds of organic compound molecules, and the resulting water and carbon dioxide bonds always release more energy than was used to break them originally. That’s why burning organic compounds produces energy and is exothermic.
Question 15.
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion (A): A solution containing a mixture of acetic acid and sodium acetate maintains a constant value of pH on addition of small amounts of acid or alkali.
Reason (R): A solution containing a mixture of acetic acid and sodium acetate acts as a buffer solution around pH 4.75.
Select the most appropriate answer from the options given below:
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is not the correct explanation of A.
(C) A is true but R is false.
(D) A is false but R is true.
Answer:
(A) Both A and R are true and R is the correct explanation of A
Question 16.
Given below are two statements labelled as Assertion (A) and Reason (R) Assertion (A): Fluorine exists only in -1 oxidation state.
Reason (R): Fluorine has 2s22p5 configuration.
Select the most appropriate answer from the options given below:
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is not the correct explanation of A.
(C) A is true but R is false.
(D) A is false but R is true.
Answer:
(B) Both A and R are true but R is not the correct explanation of A.
Explanation:
It is correct that fluorine exists only in -1 oxidation state because it has ls22p5 electronic configuration and thus shows only -1 oxidation state in order to complete its octet. Hence, both assertion and reason are true and reason is not the correct explanation of assertion.
Section-B
This section contains 5 questions with internal choice in one question, The following questions are very short answer type and carry 2 marks each.
Question 17.
Find energy of each of the photons which:
(i) corresponds to light of frequency 3 × 10
15
Hz
(ii) have wavelength of 0.50 A
Answer:
(i) Given v = 3 × 10
15
Hz
E = hv = 6.626 × 10
-34
J s × 3 × l0
15
s
-1
= 1.987 × 10
-18
J
(ii) Given λ = 0.50 Å = 0.5 x 10
-10
m
E = \(\frac{h c}{\lambda}=\frac{6.626 \times 10^{-34} \mathrm{~J} \mathrm{~s} \times 3 \times 10^8 \mathrm{~ms}^{-1}}{0.5 \times 10^{-10} \mathrm{~m}}\)
= 3.98 × 10
-15
J
![]()
Question 18.
What is the significance of the terms ‘isolated gaseous atom’ and ‘ground state’ while defining ionization enthalpy and electron gain enthalpy?
Answer:
In the gaseous state, the atoms are widely separated therefore, interatomic forces are minimum. It is because of this reason the term ‘isolated gaseous atom’ has been included in definition of ionisation enthalpy and electron gain enthalpy. The term ‘ground state’ here means that the atom must be present in the most stable state, i.e., the ground state. Therefore, for comparison purposes, the ionisation enthalpies and electron gain enthalpies of gaseous atoms must be determined in their respective ground states.
Question 19.
(a) Define the term chemical bond.
(b) What are its different types?
Answer:
(a) The attractive forces which hold the constituent atoms in molecules of species in lattices etc., is called a chemical bond.
(b) They are of the following types:
- Electrovalent or ionic bond
- Covalent bond
- Coordinate or dative bond
- Metallic bond
- Hydrogen bond
- van der Waals forces.
Question 20.
(i) Why is an organic compound fused with sodium for testing nitrogen, halogens and sulphur?
(ii) Under what conditions can the process of steam distillation used?
OR
(i) What is electromeric effect?
(ii) Compare it with hyperconjugation effect.
Answer:
- On fusing with sodium metal, the elements present in an organic compound are converted from covalent form into the ionic form.
- Steam distillation is used to purify the substances which are steam volatile and water and the liquid are not miscible with each other.
OR
-
Electromeric Effect (E effect):
It is a temporary effect. It is defined as the complete transfer of a shared pair of p-electrons to one of the atoms joined by a multiple bond on the demand of an attacking reagent. - Whereas hyperconjugation effect involves delocalisation of s electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The s electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unsha red p orbital. Hyper conjugation is permanent effect whereas electromeric effect is temporary.
Question 21.
(i) How will you convert: ethyne to but-2-yne
(ii) Draw the geometrical isomers of 2,3-dichlorobut-2-ene
Answer:
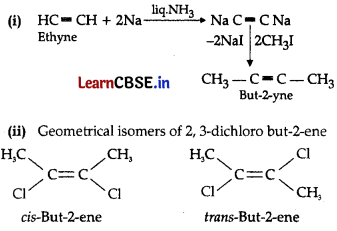
Section-C
This section contains 7 questions with internal choice in one question. The following questions are short answer type and carry 3 marks each. (No internal choice given in Board SQP)
Question 22.
(a) Write the empirical formulas of the following:
(i) Glucose, C
6
H
12
O
6
(ii) Borazine, B
3
N
3
H
6
(b) Calculate the amount of COz that could be produced when: 1 mole of carbon is burnt in 16 g of dioxygen.
(c) Calculate the number of moles in 5.0 L of 0.75 M Na
2
CO
3
.
Answer:
(a) Empirical formula-
- Glucose- CH 2 O
- Borazine- BNH 2
(b) Amount of O
2
produced from 44 g of CO
2
is 32g. i.e., 32 g of O
2
is produced from 44 g of CO
2
16g of O
2
will be produced from = \(\frac{44}{32}\) × 16 = 22g of CO
2
.
(c) Number of moles = Molarity × Volume of of Na
2
CO
3
solution in litre
= 0.75 × 5
= 3.75 mol
![]()
Question 23.
(i) Give number of electrons in the species H
2
–
, O
2
–
.
(ii) Using s, p, d notation designate the orbital:
(a) n = 3,l = l,m = 0
(b) n = 1, l = 0
(iii) List magnetic and azimuthal Quantum numbers for 3d-orbitals.
Answer:
(i) H
2
–
= 1, O
2
–
= 17
(ii) (a) 3py , (b) 1s
(iii) For 3d orbital, l = 2, m
l
= +2, +1, 0,-1, -2
Question 24.
If water vapour is assumed to be a perfect gas, molar enthalpy change for vaporisation of 1 mol of water at 1 bar and 100°C is 41 kj mol
-1
. Calculate the internal energy change, when:
(i) 1 mol of water is vaporized at 1 bar pressure and 100°C.
(ii) 1 mol of water is converted into ice.
Answer:
H
2
O(l) → H
2
O(g)
∆n
(g)
= 1 – 0 = 1
∵ ∆H = ∆U + ∆n
g
RT
or ∆U = ∆H – ∆n
g
RT
Given, ∆H = 41 KJ mol
-1
T = 100 + 273 = 373 K
∴ ∆U = 41 KJ mol
-1
– 1 × 8.3 × 10
-3
mol
-1
K
-1
× 373K
= 37.904 KJ mol
-1
(ii) H
2
O(l) → H
2
O(s)
There is negligible change in volume.
So, p∆V = ∆n
g
RT = 0
∴ ∆H = ∆U
or ∆U = 41 KJ mol
-1
Question 25.
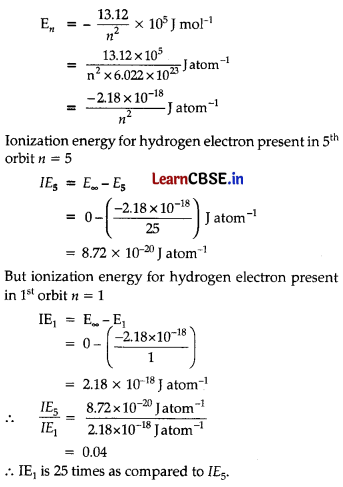
How much energy is required to ionise a H-atom if the electron occupies n = 5 orbit? Compare your answer with the ionization enthalpy of H-atom (energy required to remove the electron from n = 1 orbit).
Answer:
Energy for a hydrogen electron present in a particular energy shell
Question 26.
(a) Give the direction in which the reaction would proceed if Q
p
= K
c
(b) The value of ∆G
θ
for the phosphorylation of glucose in glycolysis is 13.8 kj/mol. Find the value of K
c
at 298 K.
(c) Hydrolysis of sucrose gives, Sucrose + H
2
O ⇌ Glucose + Fructose
Equilibrium constant Kc for the reaction is 2 × 10
13
at 300 K. Calculate ∆G
θ
at 300 K.
Answer:
(a) If Q
c
= K
c
, the reaction is in state of equilibrium.
(b) Given,
∆G
θ
= 13.8 kj/mol = 13.8 × 10
3
J/mol
T = 298 K
R = 8.314 J mol
-1
k
-1
K
c
= ?
∵ ∆G° = – RT In K
c
In K
c
= – \(\frac{\Delta \mathrm{G}^{\circ}}{\mathrm{RT}}\)
= \(\frac{-13.8 \times 10^3 \mathrm{~J} / \mathrm{mol}}{8.314 \mathrm{Jmol}^{-1} \mathrm{~K}^{-1} \times 298 \mathrm{~K}}\)
= – 5.569
or K
c
= e
-5.569
= 3.81 × 10
-3
(c) Given,
K
c
= 2 × 10
13
,
T = 300K,
∆G° = ?
∵ ∆G° = – RT in K
c
= – 8.314 J mol
-1
K
-1
× 300 K × In (2 × 10
13
)
= – 7.64 × l0
4
J mol
-1
![]()
Question 27.
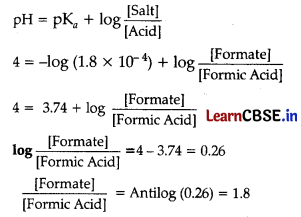
What should be the ratio of concentrations of formate ion and formic acid in a buffer solution so that its pH should be 4? Around what pH will this buffer have maximum buffer capacity? K
a
= 1.8 × 10
-4
Answer:
The buffer capacity of this solution would be maximum near the pK0 of the acid.
∴ For maximum buffer capacity
pH = pK
a
= -log K
a
= -log(1.8 × 10
-1
) = 3.74
Question 28.
Whenever a reaction between an oxidizing agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. Justify this statement giving three illustrations.
Answer:
(a) C = Reducing agent
O
2
= Oxidising agent

So, when carbon (reducing agent) is taken in excess, a compound, i.e., CO of lower oxidation state is obtained, if 02 (oxidising agent) is taken in excess, a compound (C02) of higher oxidation state is formed.
(b) P
4
= Reducing agent
Cl
2
= Oxidising’agent

Excess of oxidising agent (Cl
2
) forms a compound having higher oxidation state.
(c) Na = Reducing agent
O
2
= Oxidising agent

Section-D
The following questions are case -based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Question 29.
Read the following passage and answer the following questions:
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic Table. For example, within a period, chemical reactivity tends to be high in Group 1 metals, lower in elements towards the middle of the table, and increases to a maximum in the Group 17 non-metals. Likewise, within a group of representative metals (say alkali metals) reactivity increases on moving down the group, whereas within a group of non-metals (say halogens), reactivity decreases down the group. There are numerous physical properties of elements such as melting and boiling points, heats of fusion and vaporization, energy of atomization, etc. which show periodic variations.
(a) What will be trend of metallic character across the period?
(b) First Ionization enthalpy of Sodium metal is lower than Mg. why?
(c) How will you justify that all mention elements are isoelectronic species?
O
2-
, F
–
, Na
+
and Mg
2+
OR
Why ionization enthalpy of nitrogen is higher than carbon?
Answer:
(a) The metallic character across the period decreases because tendency of elements to lose the electrons decreases and electron gain enthalpy increases. (1)
(b) Electronic configuration of Na is Is 2 ,2s 2 , 2p 6 , 3s 1 Electronic configuration of Mg is 1s 2 , 2s 2 ,2p 6 , 3s 2 Na has one electron in 3s orbital, so, less energy will be required to extract out an electron from 3s orbital whereas Mg has two electrons in 3s orbital, so, High amount of energy will be required to extract out an electron from 3s orbital.
(c) Number of electron in O
2
–
= 8 + 2 = 10
Number of electron in F
–
= 9 + 1 = 10
Number of electron in Na
+
= 11 – 1 = 10
Number of electron in Mg
2+
= 12 – 2 = 10
OR
Nitrogen E.C is 1s
2
2s
2
2p
3
, it is having half filled p-orbitals which needs high energy to remove an electron whereas Carbon has two electrons in p orbital which is unstable and needs less energy to remove an electron.
![]()
Question 30.
Read the following passage and answer the following questions:
AU Measurements for chemical reactions, heat absorbed at constant volume, is measured in a bomb calorimeter. Here, a steel vessel (the bomb) is immersed in a water bath. The whole device is called calorimeter. The steel vessel ‘ is immersed in water bath to ensure that no heat is lost to the surroundings. A combustible substance is burnt in pure dioxygen supplied in the steel bomb. Heat evolved during the reaction is transferred to the water around the bomb and its temperature is monitored. Since the bomb calorimeter is sealed, its volume does not change i.e., the energy changes associated with reactions are measured at constant volume. Under these conditions, no work is done as the reaction is carried out at constant volume in the bomb calorimeter. Even for reactions involving gases, there is no work done as AV = 0. Temperature change of the calorimeter produced by the completed reaction is then converted to qV, by using the known heat capacity of the calorimeter with the help of equation.
(a) Define Calorimetry.
(b) Write a chemical equation in which ∆H and ∆U are equal.
(c) What is Extensive and intensive property?
Answer:
(a) The technique used for measure energy changes associated with chemical or physical processes by an experimental technique called calorimetry.
(b) H
2
(g) + I
2
(g) → 2HI(g)
∴ ∆H = ∆U + ∆n
g
RT
∆n
g
= n
p
– n
r
For this reaction, ∆n = 0
∴ ∆H = AU
(c) In thermodynamics, a distinction is made between extensive properties and intensive properties. An extensive property is a property whose value depends on the quantity or size of matter present in the system. For example, mass, volume, internal energy, enthalpy, heat capacity, etc. are extensive properties. Those properties which do not depend on the quantity or size of matter present are known as intensive properties. For example temperature, density, pressure etc.
are intensive properties. A molar property, %m, is the value of an extensive property % of the system for 1 mol of the substance. If n is the amount of matter, = n is independent of the amount of matter. Other examples are molar volume .
OR
For isothermal (T = constant) expansion of an ideal gas into vacuum; w = 0 since pex = 0. Also, Joule determined experimentally that q = 0; therefore, ∆U = 0 , ∆ = + U q w can be expressed for isothermal irreversible and reversible changes as follows:
Section-E
The following questions are long answer type and carry 5 marks each. All questions have an internal choice.
Question 31.
Attempt any five of the following:
(a) Structures of molecules of two compounds are given below:
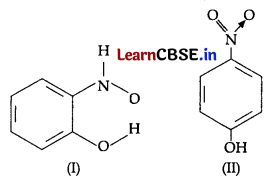
Which of the above two compounds will show higher boiling point?
(b) Axial bonds of PC1
5
are longer than equatorial bonds. Why? Give reason.
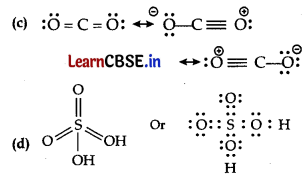
(c) Write the resonance structure for CO
2
.
(d) Draw Lewis structure of H
2
SO
4
.
(e) Why KHF2 exist but KHC1
2
does not?
(f) Define the bond length.
(g) Explain why BeH
2
molecule has a zero dipole moment although the Be-H bonds are polar?
Answer:
(a) Compound II (p-nitrophenol) has higher boiling point. In p-nitrophenol, molecular association takes place due to extensive hydrogen bonding between the molecules (intermolecular hydrogen bonding). Now, due to the intermolecular hydrogen bonding, more energy needs to be supplied to vaporize the compound as there are extra bonds other than those present that hold the molecule. Therefore, it has higher boiling point. In compound I (o-nirophenol), the intramolecular hydrogen bond results in the cyclisation of the molecule and prevents their association. Consequently, less energy needs to be supplied to vaporize the compound.
(b) In PC1 5 , there are three equatorial bonds and two axial bonds. The axial bonds are longer than equatorial bonds. The two axial bonds are at 90° to each other because the axial Cl atoms suffer from more repulsion than the equatorial Cl atoms. As a result, the axial Cl atoms tries to reside far away from the equatorial Cl atoms and hence axial bonds are longer than equatorial bonds.
(e) KF forms H-bonds with HF whereas KC1 cannot form H-bond with HC1.
Hence, KHF
2
can dissociate to give HF
2-
ion and therefore, KHF
2
exists. However, KHC1
2
cannot dissociate to HC1
2-
ion and therefore, KHC1
2
does not exist. (1)
(f) It is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. OR It is defined as the average distance between the centres of the nuclei of the two bonded atoms in a molecule.
(g) BeH
2
is a linrae molecule
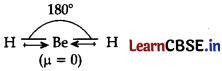
In this molecule, the two equal bond dipoles point in opposite directions and cancel the effect of each other. Hence, BeH
2
molecule has a zero dipole moment (m = 0).
Question 32.
(a) Why is impure glycerol purified by distillation under reduced pressure?
(b) Write a short note on differential extraction.
(c) Can we separate two liquids A (boiling point 353 K) and B (boiling point 365 K) present in a mixture by simple distillation?
OR
(a) What are the properties of resonance hybrid?
(b) (CH
3
)
3
C
+
(tert-Butyl carbocation) is more stable than (CH
3
)
2
C
+
H (sec-Propyl carbocation). Why?
Answer:
(a) At normal pressure, the boiling point of glycerol is 563 K but it decomposes on this temperature. Therefore, simple distillation cannot be used. Under a reduced pressure of 12 mm Hg, the glycerol can be distilled at 453 K without decomposition.
(b) When an organic compound is present in an aqueous medium, it is separated by shaking it with organic solvent in which it is more soluble than in water. The aqueous solution is mixed with organic solvent in a separating funnel and shaken for sometimes and then allowed to stand for some time. When organic solvent and water. form two separate layers, the lower layer is run out by opening the tap of funnel and organic layer is separated. The process is repeated several times and pure organic compound is separated.
(c) No, because in simple distillation, vapours of both the liquids will be formed simultaneously and will condense together in receiver as the difference between the boiling points is very less. They can be separated by fractional distillation.
OR
(a) Following are the properties of resonance hybrid:
-
Stability:
A resonance hybrid is more stable than any of the contributing structures. Greater the number of equivalent contributing structures of a molecule, greater will be the stability of the resonance hybrid. -
Resonance energy:
It is equal to the difference between the energy of the resonance hybrid and of the most stable resonating structure. More the resonance energy of a molecule, greater is the stability due to resonance, e.g., the resonance energy of benzene is 151 kJ mol -1 . It means benzene is more stable than any of its contributing structures by 151 kJ mol -1 -1. -
Bond lengths:
The bond lengths of resonance hybrid are always different from that of any of the contributing structures e.g., in benzene.
(b) Tert-Butyl carbocation is more stable than sec- propyl carbocation due to hyperconjugation. Similarly, hydrogen of other two methyl groups can undergo hyperconjugation resulting in 10-resonance structures. However, in case of sec- propyl carbocation, only 7-resonance structures are possible.
![]()
Question 33.
How do you account for the formation of ethane during chlorination of methane?
OR
(a) (i) Write the chemical reactions for the Ozonolysis.
(ii) Write IUPAC names of the products obtained the ozonolysis of 3,4-dimethyl hept-3-ene.
(b) (i) Arrange the following in the decreasing order of acidic character.
• C
2
H
4
C
2
H
6
C
2
H
2
• CH
3
— C = CH, C
2
H
2
CH
3
— C = C — CH
3
.
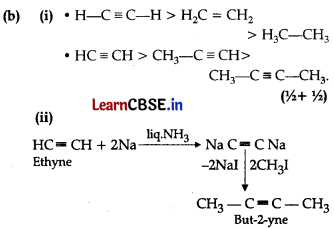
(ii) How will you convert: ethyne to but-2-yne, ,
(iii) How many structural isomer are possible for pentane? Draw the geometrical isomers of 2, 3-dichlorobut 2-ene
Answer:
Chlorination of methane proceeds through a free radical chain mechanism. The whole reaction takes place in the given three steps.
Step 1: Initiation:
The reaction begins with the homolytic cleavage of Cl – Cl bond as:
![]()
Step 2: Propagation:
In the second step, chlorine free radicals attack methane molecules and break down the C-H bond to generate methyl radicals as:
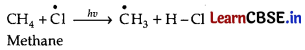
These methyl radicals react with other chlorine free radicals to form methyl chloride along with the liberation of a chlorine free radical.
![]()
Hence, methyl free radicals and chlorine free radicals set up a chain reaction. While HC1 and CH3C1 are the major products formed, other higher halogenated compounds are also formed as:

Step 3: Termination:
Formation of ethane is a result of the termination of chain reactions taking place as a result of the consumption of reactants as:
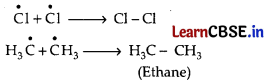
Hence, by this process, ethane is obtained as a by-product of chlorination of methane.
OR
(a) (i) Alkenes or alkynes add ozone to form ozonoid which on hydrolysis in the presence of zinc give aldehyde or ketone or carboxylic acid.
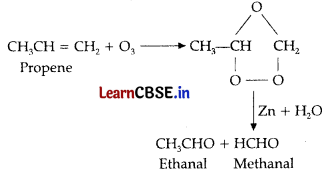
(ii) The products obtained by the ozonolysis of 3,4-dimethy hept-3-ene are butanone and pentane-2-one.
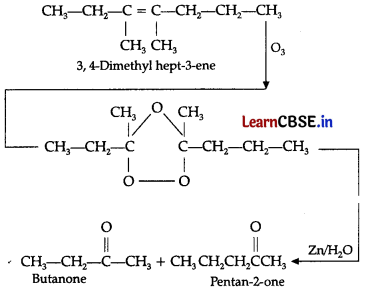
(iii) C
2
H
12
is on organic compound having five carbon atoms. It has three structural isomers like n-pentane.
Iso-pentane and Neo-pentane.