Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Term 2 Set 7 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Term 2 Set 7 for Practice
Time allowed: 2 Hours
Maximum Marks: 40
General Instructions:
- All questions are compulsory.
- The question paper has three sections and 15 questions. ALL questions are compulsory.
- Section-A has 7 questions of 2 marks each; Section-B has 6 questions of 3 marks each, and Section-C has 2 case based questions of 4 marks each.
- Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
SECTION – A
Question 1.
(A) Find the value of current in the circuit given os below. (1)
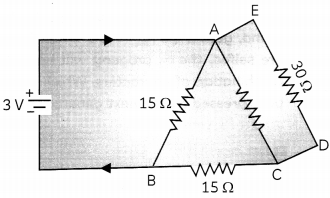
(B) You have four resistors of 8Ω each. Show how would you connect these resistor to have effective resistance of 8Ω? (1)
OR
Calculate the equivalent resistance of the following network:
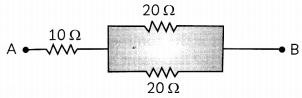
Question 2.
(A) Two carbon atoms cannot be Linked to each other by more than three covalent bonds. Why? (1)
(B) What will be the formula and electron dot structure of cyctopentane? (1)
Question 3.
Which of these organisms are capable of restoring damaged or missing cells, tissues, organs, and even entire body parts?
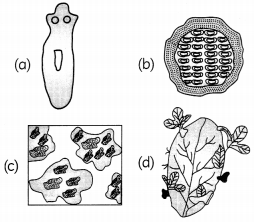
OR
Observe the diagrams shown below.
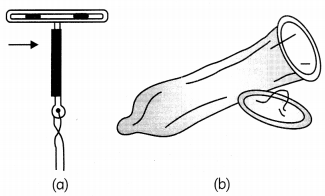
What are STDS? Which one of the contraceptive devices is used to prevent AIDS? (2)
Question 4.
The following table shows a part of the periodic table in which the elements are arranged according to their atomic numbers. (The letters given here are not the chemical symbols of the elements):
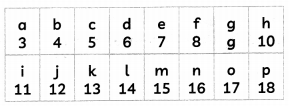
Giving reasons, answer the following:
(A) Which element has a bigger atom, ‘a’ or ‘r’? (1)
(B) Which element has a higher valency, ‘k’ or ‘o’? (1)
Question 5.
(A) In what form the 10% of energy is transferred from one trophic Level to the next in an ecosystem? (1)
(B) According to 10% law, only 10% of the energy entering a particular trophic level is available for transfer to the organisms of the next trophic level. What happens to the 90% energy? (1)
Question 6.
Identify the functional group present in the given sets of compounds.
(A) Propanal
(B) Propanoic acid
(C) Propanone
(D) Propanol (2)
Question 7.
Observe the cross shown below and predict what would be the ratio of F
2
progeny? Explain. (2)
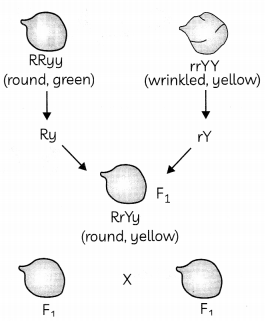
OR
Two pea plants one with round green seeds (RRyy) and another with wrinkled yellow (rrYY) seeds produce F
2
progeny that have round, yellow (RrYy) seeds. When F
1
plants are selfed, the F
2
progeny will have a new combination of characters. Which traits will be expressed by the next generation? (2)
SECTION – B
Question 8.
The below diagram is a depiction of the human female reproductive system.
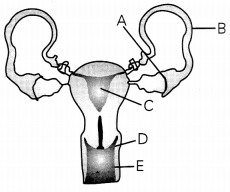
Label all the parts and write down why part B is ligated and sutured. (3)
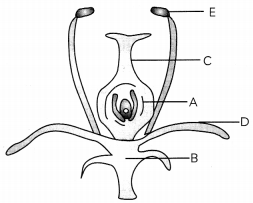
OR
Observe the figure given alongside and label A, B, C, D and E and write the function of D. (3)
Question 9.
(A) What is the commercial unit of electrical energy? Represent it in terms of joules. (1)
(B) A battery of 9V is connected in series with resistors of 0.2 , 0.3 , 0.4,0.5 and 12 ohms respectively. How much current will flow through a 12 ohm resistor? (1)
(C) Why are coils of electric toasters and electric iron made of an alloy rather than a pure metal? (1)
Question 10.
List two contrasting visible characters of garden pea which Mendel used for his experiment. (3)
OR
Explain how Mendel interpreted his results to show that the traits may be dominant or recessive. (3)
Question 11.
(A) Draw a diagram to show magnetic field due to a bar magnet in a given region. (1)
(B) Mention the shape of the magnetic field lines around a current carrying straight conductor. (1)
(C) What is the role of the two conducting stationary brushes in a simple electric motor? (1)
Question 12.
(A) On detailed reserach, it was found that, in human beings, the statistical probability of getting either a male or female child is 50 : 50. Give a suitable explanation. (1½)
(B) Enlist the differences between acquired and inherited traits. Support your answer with an example. (1½)
Question 13.
What are decomposers? What will be the consequence of their absence in an ecosystem? (3)
SECTION – C
False
Question 14.
The magnetic field lines of an infinite wire are circular and centered at the wire and they are identical in every plane perpendicular to the wire as shown in the figure.
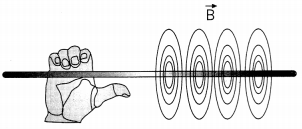
Since the field decreases with distance from the wire, the spacing of the field lines must increase correspondingly with distance. The direction of this magnetic field may be found with a second form of the right-hand rule. If you hold the wire with your right hand so that your thumb points along the current, then your fingers wrap around the wire in the same sense as B.
(A) A vertical wire carries an electric current out of the page. What is the direction of the magnetic field at point P located to the west from the wire? (1)
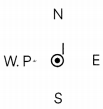
(B) A current carrying conductor is held in exactly vertical direction. In order to produce a clockwise magnetic field around the conductor, the current should be passed in the conductor. What will be the direction of the current? (1)
(C) What are the characteristics of magnetic field lines? (1½)
OR
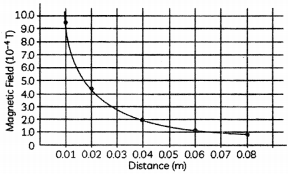
A student plotted a variation of magnetic field around a straight current carrying wire and the distance from the wire where the magnetic field is measured. (1½)
OR
Study the graph below and find out the relationship between magentic field and distance.
A positive charge is moving towards a person. What will be the direction of magnetic field lines? (2)
Question 15.
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electron shells but different number of electrons in their outermost shells. It was found that elements A and G combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during cooking. Oxides of elements A and B are basic in nature while those of elements E and F are acidic. The oxide of element D is, however, almost netural. Based on the above information, answer the following questions:
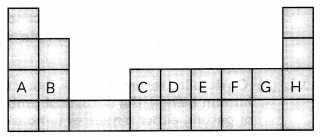
(A) To which group or period of the periodic table do these elements belong? (1)
(B) What would be the nature of compound formed by a combindation of elements B and F? (1)
(C) (i) Which two of these elements could definitely be metals and which would be non metals? (1)
(ii) Which one of the eight elements is most likely to be found in gaseous state at room temperature? (1)
OR
(i) Identify the letter which represents metalloid from the above elements. Name that metalloid. (1)
(ii) Show the formation of a diatomic molecule of ‘G’ and predict its nature. (1)