Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Term 2 Set 6 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Term 2 Set 6 for Practice
Time allowed: 2 Hours
Maximum Marks: 40
General Instructions:
- All questions are compulsory.
- The question paper has three sections and 15 questions. ALL questions are compulsory.
- Section-A has 7 questions of 2 marks each; Section-B has 6 questions of 3 marks each, and Section-C has 2 case based questions of 4 marks each.
- Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
Section – A
Question 1.
Do all variations in a species have equal chances of surviving in the environment in which they find themselves?
OR
Mendel performed his first set of experiments on the pea plants. Why did Mendel choose pea plants as an experimental subject? (2)
Question 2.
A current through a horizontal power line flows in west to east direction.
(A) What is the direction of the magnetic field at a point directly above it and at a point directly below it? (1)
(B) Name the rule used to determine:
(i) The direction of force when a current carrying wire is placed in a strong magnetic field. (1/2)
(ii) Magnetic field in a current carrying conductor. (1/2)
Question 3.
(A) What is an ecosystem? (1)
(B) The number of trophic levels in a food chain is limited. Comment on this statement. (1)
OR
Which groups of organisms are not constituents of a food chain?
(A) Plankton, man, fish, grasshopper (1)
(B) Wolf, grass, snake, tiger (1)
Question 4.
The figure shows the pattern of magnetic field lines through a circular loop.
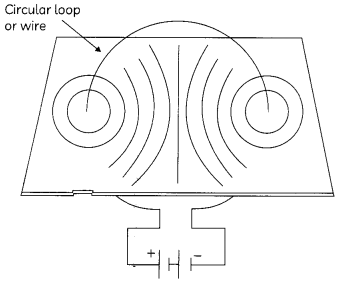
Mark the direction of:
(A) electric current in loop (1)
(B) magnetic field lines (1)
Question 5.
From the dihybrid cross shown below answer the following questions:
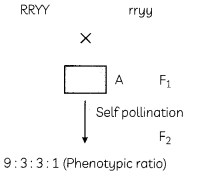
(A) Write the type of seeds produced in F
1
generation. (1/2)
(B) Write the type of seeds that were 9 : 3 : 3 :1 in ratio respectively. (1/2)
(C) Show the cross obtained after self pollination of F
1
progeny. (1)
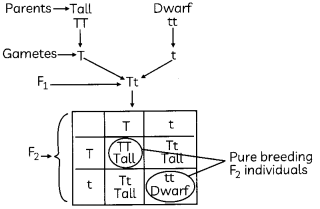
OR
Given below is a depiction of Mendele’s experiment with the pea plants. An enclosed table shows the inheritance pattern in the F
2
generation. What would be the ratio of the next generation, if pure breeding of F
2
individuals (encircled) is done? (2)
Question 6.
Figure shows a 240 V AC mains circuit to which a number of appliances are connected and switched on.
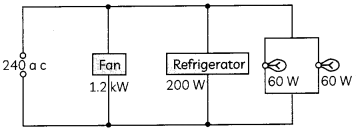
(A) Calculate the power supplied to the circuit. (1)
(B) Calculate
(i) the current through the refrigerator,
(ii) the energy used by the fan in 3 hours,
(iii) the resistance of the filamen of one lamp. (1)
Question 7.
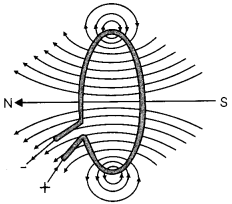
(A) What do the magnetic field lines depict in given figure? (1/2)
(B) State whether an alpha particle will experience any force in a magnetic field, if.
(i) it is placed in the field at rest.
(ii) it moves in the magnetic field parallel to field lines.
(iii) it moves in the magnetic field perpendicular to field Lines (1½)
Section B
Question 8.
Give reasons for the following:
(A) Non-metals have a tendency to form anions. (1)
(B) Cs atom is larger than Li atom. (1)
(C) Metals have a tendency to lose electrons. (1)
OR
(A) Give reasons for the following:
(i) Aluminium and Zinc have a tendency to form amphoteric oxides. (1)
(ii) Size of an element decreases on moving along the period. (1)
(B) An element ‘A’ belongs to the second period and group 13 of the Periodic Table. Find out the valency of A. (1)
Question 9.
What is placenta? Mention its role during pregnancy. (3)
OR
(A) Write the correct sequence of reproductive stages seen in flowering plants. (1½)
(B) Why does the number of chromosomes in parents and offsprings of a particular species remains constant? (1½)
Question 10.
Draw the diagram of a flower and label the four whorls. Write the names of gamete producing organs in the flower. (3)
Question 11.
The formula of four organic compounds are given below:
![]()
(A) Which one of the compounds is a saturated hydrocarbon? (1)
(B) Identify the organic acid from among the following and give its structural formula. (1)
(C) Which among (a) and (d) will be more reactive? Why? (1)
Question 12.
(A) Why are crop fields known as artificial ecosystems? (1½)
(B) What are the advantages of cloth bags over plastic bags during shopping? (1½)
Question 13.
Ashish observed the ears of all the students in the class and prepared a list of students having free and attached earlobes:
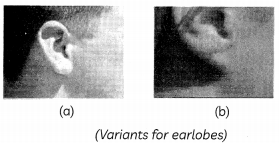
After calculations he found that 70% of students of his class have free earlobes whereas only 30% have attached ear lobes.
(A) Which ear lobes are dominant – free earlobes or attached earlobes? (1)
(B) Suggest a possible rule for the inheritance of earlobe types. (1)
(C) What are dominant traits? (1)
Section – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (A, B and C).
Parts A and B are compulsory. However, an internal choice has been provided in part C.
Question 14.
Pin was a very curious child, The wanted to establish a relationship between potential difference across to conductor and current flowering through it. For this he sets up an electric circuit comprising a nichrome wire, ammeter A, voltmeter V, plug key K and 5 cells of same voltage (let’s say 1.5 V each)
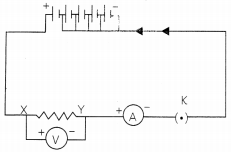
First, he used one cell as sources of current in the circuit and noted the reading of ammeter and voltmeter. Then he repeated the above steps using two, three, four and five cells, and noted the reading which are given in the table alongside:

(A) Plot a graph between V and 1 and calculate the resistance of that resistor. (1)
(B) Who gave the relationship between the current, I, flowing in a metallic wire and the potential difference across it terminals. Define the law used to calculate the resistance of that resistor. (1)
(C) Does the phenomenon/law hold good under all conditions? Comment. (2)
OR
Two identical resistors each of resistance 12 ohm are connected, (i) in series (ii) in parallel, in turn to a battery of 6 V. Calculate the ratio of power consumed in the combination of resistors in the two cases. (2)
Question 15.
The table represents a periodic table used in chemistry. Use the below periodic table to answer the following questions:
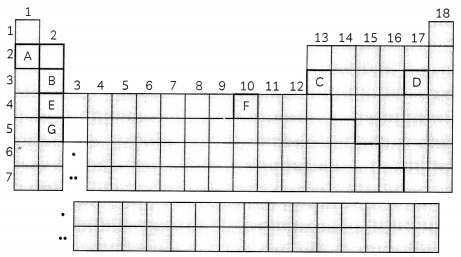
(A) Identify the element Located in group 2A and 4th period? Identify the period and group of element C? (1)
(B) Which element has the lowest electronegativity? (1 )
(C) Arrange (B
2+
, C
3+
, D
-1
) ions in order of increasing ionic radius. (2)
OR
Write the electronic configuration of element D and its anion D
–
. Compare its properties. (2)