Students can find that CBSE Previous Year Question Papers Class 10 Science with Solutions and CBSE Class 10 Science Question Paper 2020 (Series: JBB/3) effectively boost their confidence.
CBSE Class 10 Science Board Question Paper 2020 (Series: JBB/3) with Solutions
Time allowed: 3 hours
Maximum marks: 80
General Instructions
-
Question Paper comprises three sections – A, B and C.
There are 30 questions in the question paper. All questions are compulsory. - Section A – questions no. 1 to 14 – all questions or part thereof are of one mark each. These questions comprise multiple choice questions (MCQ), very short answer (VSA), and Assertion-Reason type questions. Answers to these questions should be given in one word or one sentence.
- Section B – questions no. 15 to 24 are short answer type questions, carrying 3 marks each. Answers to these questions should not exceed 50 to 60 words.
- Section C – questions no. 25 to 30 are long answer type questions, carrying 5 marks each. Answers to these questions should not exceed 80 to 90 words.
- Answers should be brief and to the point. Also the above mentioned word limit be adhered to as far as possible.
- There is no overall choice in the question paper. However, an internal choice has been provided in some questions in each Section. Only one of the choices in such questions has to be attempted.
- In addition to this, separate instructions are given with each section and question, wherever necessary.
SET – I Code No. 31/3/1
Section – A
Question 1.
How are covalent bonds formed?
Answer:
Covalent bonds are formed by sharing of electrons between the two atoms in order to acquire inert gas configuration.
Question 2.
Define Tyndall’s effect.
OR
The ciliary muscles of a normal eye are in their
(i) most-relaxed
(ii) most-contracted state. In which of the two cases is the focal length of the eye lens more?
Answer:
The scattering of a beam of light is called the Tyndall effect. Tyndall effect can be observed when sunlight passes through the canopy of a dense forest. In a dense forest, the mist contains tiny droplets of water, which act as particles of colloid dispersed in air.
OR
The food length of the eye-lens is more in most-relaxed state.
![]()
Question 3.
Answer question numbers 3(a)-3(d) on the basis of your understanding of the given paragraph and the related studied concepts.
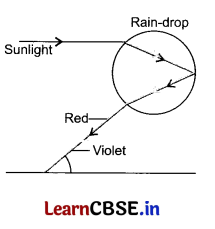
Rainbow is an optical natural spectrum, produced by the nature in the sky, in the form of a multicoloured arc. The rainbow is formed due to the dispersion of sunlight by water droplets suspended in the atmosphere after rainfall.

They refract and disperse the incident sunlight, then reflect it internally and finally refract. Due to the dispersion of light and internal reflection, different colours reach the observer’s eye.
(a) Define the phenomenon of dispersion.
(b) What are the different colours seen in a rainbow?
(c) What do you mean by internal reflection?
(d) Draw a labelled ray diagram to show the formation of a rainbow.
Answer:
(a) Dispersion of light When a ray of white light on passing through a prism split into seven colours then this phenomenon is known as dispersion.
(b) Seven different colours are seen in the rainbow which are violet, indigo, blue, green, yellow, orange and red.
(c) When a ray of light is reflected back within a medium then it is known as internal reflection.
(d)
Question 4.
Questions number 4(a) to 4(d) are based on the table given below. Study the table in which the levels of Thyroid Stimulating Hormone (TSH) in women are given and answer the questions that follow on the basis of understanding of the following paragraph and the related studied concepts.
| Age Range | Normal (mU/L) | Low (mU/L) |
| 18-29 years | 0.4-2.34 mU/L | < 0.4 mU/L |
| 30-49 years | 0.4-4.0 mU/L | < 0.4 mU/L |
| 50-79 years | 0.46-4.68 mU/L | < 0.46 mU/L |
Women are at greater risk for developing abnormal TSH levels during menstruation, while giving birth and after going through menopause. Around 5% of women in the United States have some kind of thyroid problem compared to 3% of men. Despite claims that high TSH increases your risk for heart disease, a 2013 study found no link between high TSH and heart diseases. But a 2017 study showed that older women are especially at risk for developing thyroid cancer if they have high TSH levels along with thyroid nodules.
(a) A 35 year old woman has TSH level 6.03 mU/L. What change should she bring in her diet to control this level?
(b) When do women face a greater risk of abnormal TSH level?
(c) State the consequences of low TSH level.
(d) Name the mineral that is responsible for synthesis of hormone secreted by thyroid gland.
Answer:
(a) The woman should include in her diet food rich in iodine, vitamin A, zinc and protein.
(b) Women are at greater risk for developing abnormal TSH levels during menstruation, while giving birth and after going through menopause.
(c) Consequences of low TSH level.
(i) weight-gain even after taking normal diet;
(ii) dry qnd scaly skin;
(iii) depression and
(iv) hairfall.
(d) Iodine is responsible for synthesis of thyroxine hormone secreted by thyroid gland.
Question 5.
The sky appears dark to passengers flying at very high altitudes mainly because:
(a) Scattering of light is not enough at such heights.
(b) There is no atmosphere at great heights.
(c) The size of molecules is smaller than the wavelength of visible light.
(d) The light gets scattered towards the earth.
Answer:
(a) Scattering of light is not enough at such heights.
Question 6.
A cylindrical conductor of length ‘V and uniform area of cross section ‘A’ has resistance ‘R’. The area of cross section of another conductor of same material and same resistance but of length ‘2l’ is
(a) \(\frac { A }{ 2 }\)
(b) \(\frac { 3A }{ 2 }\)
(c) 2A
(d) 3A
Answer:
(c) 2A
![]()
Question 7.
The maximum resistance which can be made using four resistors each of resistance 1/2 Ω is: 1
(a) 2 Ω
(b) 1 Ω
(c) 2.5 Ω
(d) 8 Ω
Answer:
(a) 2 Ω
Question 8.
A diagram of traditional water harvesting system is given below:
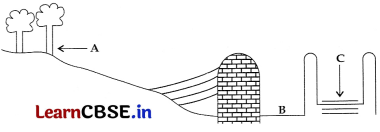
The statement which defines the system and its parts is –
(a) This is an ideal setting of the Khadin system and A = Catchment area; B = Saline area and C = Shallow dugwell
(b) This is an ideal setting of the Shallow dugwell system and A = Catchment area; B = Saline area and C = Khadin
(c) This is an ideal setting of Catchment area and A = Khadin, B = Saline area and C = Shallow dugwell
(d) This is showing Saline area and A = Catchment area; B = Khadin and C = Shallow dugwell
OR
The major ill effect of mono culture practice in forests is on the –
(a) biodiversity which faces large destruction.
(b) local people whose basic needs can no longer be met from such forests.
(c) industries.
(d) forest department.
Answer:
(a) This is an ideal setting of the Khadin system and A = Catchment area; B = Saline area and C = Shallow dugwell
OR
(a) biodiversity which faces large destruction.
Question 9.
Several factories were pouring their wastes in rivers A and B. Water samples were collected from these two rivers. It was observed that sample collected from river A was acidic while that of river B was basic. The factories located near A and B are
(a) Soaps and detergents factories near A and alcohol distillery near B.
(b) Soaps and detergents factories near B and alcohol distillery near A.
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
(d) Lead storage battery manufacturing factories near B and soaps and detergents factories near A.
Answer:
(c) Lead storage battery manufacturing factories near A and soaps and detergents factories near B.
Question 10.
In which of the following, the identity of initial substance remains unchanged?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer:
(b) Formation of crystals by process of crystallisation
Question 11.
An. aqueous solution ‘A’ turns phenolphthalein solution pink. On addition of an aqueous solution ‘B’ to ‘A’, the pink colour disappears. The following statement is true for solution ‘A’ and ‘B‘.
(a) A is strongly basic and B is a weak base.
(b) A is strongly acidic and B is a weak acid.
(c) A has pH greater than 7 and B has pH less than 7.
(d) A has pH less than 7 and B has pH greater than 7.
Answer:
(c) A has pH greater than 7 and B has pH less than 7.
Question 12.
Which of the following will undergo addition reactions?
(a) CH
4
(b) C
3
H
8
(C) C
2
H
6
(d) C
2
H
4
OR
Which of the following acids is present in tomato?
(a) Acetic acid
(b) Citric acid
(c) Oxalic acid
(d) Tartaric acid
Answer:
(d) C
2
H
4
OR
(c) Oxalic acid
Note: For questions number 13 and 14, two statements are given – one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both (A) and (R) are true and (R) is the correct explanation of the Assertion.
(b) Both (A) and (R) are true but (R) is not the correct explanation of the Assertion.
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
![]()
Question 13.
Assertion (A): Following are the members of a homologous series:
CH
3
OH, CH
3
CH
2
OH, CH
3
CH
2
CH
2
OH
Reason (R): A series of compounds with same functional group but differing by—CH
2
— unit is called a homologous series.
Answer:
(a) Both (A) and (R) are true and (R) is the correct explanation of the Assertion.
Question 14.
Assertion (A): Concave mirrors are used as rear-view mirrors in vehicles.
Reason (R): Concave mirrors produce an erect and magnified image when object is placed between P and F of the mirror.
Answer:
(d) (A) is false but (R) is true.
Section – B
Question 15.
Mention with reason the colour changes observed when:
(i) silver chloride is exposed to sunlight.
(ii) copper powder is strongly heated in the presence of oxygen.
(iii) a piece of zinc is dropped in copper sulphate solution.
Answer:
(i) Observation. The white colour of silver chloride turns grey in sunlight. This is due to the decomposition of silver chloride into silver and chlorine by light.
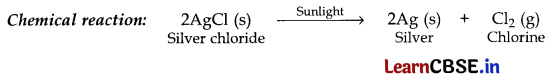
Type of reaction: Photolytic decomposition reaction
(ii) When copper powder is strongly heated in the presence of oxygen it forms a black substance Copper (II) oxide.
![]()
(iii) When a piece of zinc is dropped in copper sulphate solution, the blue colour of the copper sulphate solution starts disappearing because zinc displaces copper from copper sulphate and forms colourless zinc sulphate.
![]()
Question 16.
Complete and balance the following chemical equations:
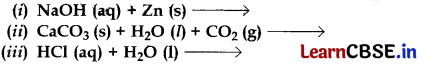
Or
During electrolysis of brine, a gas ‘G’ is liberated at anode. When this gas ‘G’ is passed through slaked lime, a compound ‘O’ is formed, which is used for disinfecting drinking water.
(i) Write formula of ‘G’ and ‘C’.
(ii) State the chemical equation involved.
(iii) What is common name of compound ‘C? Give its chemical name.
Answer:
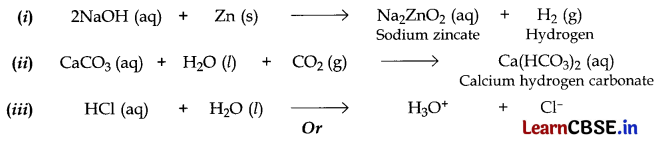
OR
(i) Formula of G = Cl
2
(Chlorine gas); Formula of C = CaOCl
2
(ii)
![]()
(iii) Common name of ‘C = Bleaching powder
Chemical name of ‘C’ = Calcium oxychloride
Question 17.
What is meant by isomerism? Why do first three members of the alkane series not show isomerism? Write the structure of two isomers of the fourth member of this series.
Answer:
• Isomerism is the existence of two or more compounds having same molecular formula but different structures.
• It is not possible for them to have different structures/only one structure is possible for each of them.
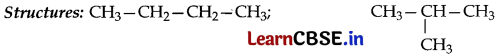
Question 18.
(a) From the given group of organisms create a food chain which is the most advantageous for Human beings in terms of energy.

(b) State the possible disadvantage if the cereal plant is growing in soil rich in pesticides.
(c) Construct a food web using the organisms mentioned above.
OR
(a) Write two harmful effects of using plastic bags on the environment. Suggest alternatives to the usage of plastic tegs.
(b) List any two practices that can be followed to dispose off the waste produced in our homes.
Answer:
(a) Cereal Plant → Goat → Human being
Since only 10% energy is transferred to the next level thus there is a gradual reduction in the amount of energy. So herbivores acquire the highest energy from the producers.
(b) Some harmful chemicals like pesticides, when absorbed by the plants through soil and water, get transferred from first trophic level to the last trophic level of the food chain. As these chemicals are non-degradable, their concentration in the bodies of living organisms at each trophic level progressively increases. This increase in the concentration of harmful chemicals in the body of living organisms at each trophic level of a food chain is called biological magnification. The level of concentration of chemicals is maximum for human beings as they are at the highest trophic level.
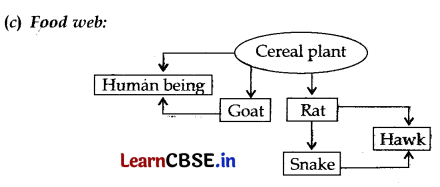
OR
(a) Harmful effects of using plastic bags on the environment
- Plastic is non-biodegradable so it will remain as such and pollute the environment
- Burning of plastic bags produces toxic gases and can block the drainage system,
- Discarded plastic bags when eaten by cows and other stray animals can block their alimentary canal and cause harm to them.
- Plastic bags when thrown in the water bodies, can cause water pollution as these do not decompose. (any two)
Alternatives to the usage of plastic bags:
- We should take our own jute or cloth bags while going for purchasing articles from the market.
- Disposable paper cups should be used for serving tea in trains instead of plastic cups.
- Shopkeepers can use paper bags instead of plastic bags.
(b) Practices that can be followed to dispose off the waste produced in our homes:
- Disposal of wastes after separating them into biodegradable and non- biodegradable waste in separate dustbins.
- Reusing plastic containers and glass containers to store kitchen items.
Question 19.
(a) State the role played by the following in the process of digestion.
(i) Enzyme trypsin
(ii) Enzyme lipase
(b) List two functions of finger like projections present in the small intestine.
Answer:
(a) (i) Enzyme trypsin breaks proteins into peptones and peptides and finally to amino acids.
(ii) Enzyme lipase breaks down emulsified fats into glycerol and finally to fatty acids.
(b) Functions of finger like projections present in the small intestine. The inner surface of small intestine has millions of tiny, finger like projections called villi. The presence of villi gives the inner walls of the small intestine a very large surface area and the large surface area of small intestine helps in the rapid absorption of digested food.
Question 20.
(a) What is fertilization?
(b) Distinguish between internal and external fertilization.
(c) What is the site of fertilization in human beings?
Answer:
(a) Union of male gamete with female gamete.
(b) Internal fertilization takes place inside the female body.
External fertilization takes place outside the female body.
(c) Fallopian tube in human female
Question 21.
A green stemmed rose plant denoted by GG and a brown stemmed rose plant denoted by gg are allowed to undergo a cross with each other.
(a) List your observations regarding
(i) Colour of stem in their F
1
progeny.
(ii) Percentage of brown stemmed plants in F
2
progeny if F
1
plants are self pollinated.
(iii) Ratio of GG and Gg in the F
2
progeny.
(b) Based on the findings of this cross, what conclusion can be drawn?
Answer:
Green stemmed rose plant = GG;
Brown stemmed rose plant = gg
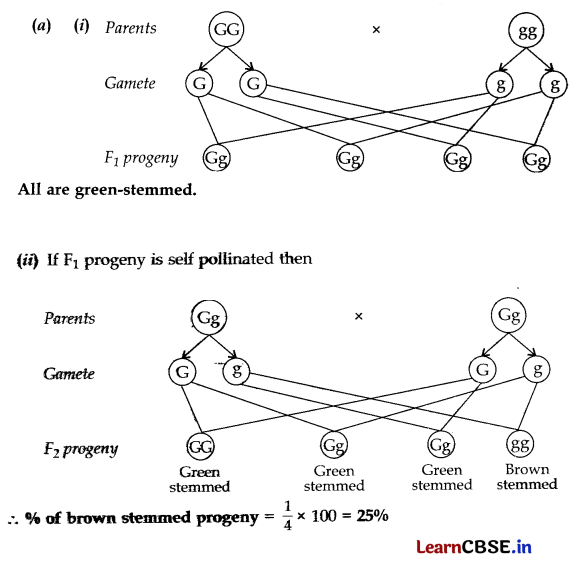
(iii) Ratio of GG and Gg in F
2
progeny = 1 : 2
(b) Conclusion. The characteristics (or traits) of an organism are determined by internal ‘factors’ which occur in pairs. Only one pair of such factors can be present in a single gamete. One of such traits is dominant over the other trait.
Question 22.
The diagram given below shows an object O and its image I.
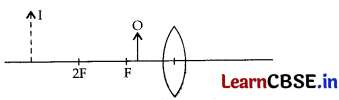
Without actually drawing the ray diagram, state the following:
(i) Type of lens (Converging/ Ehverging)
(ii) Name two optical instruments where such an image is obtained.
(iii) List three characteristics of the image formed if this lens is replaced by a concave mirror of focal length ‘f and an object is placed at a distance ‘f/ 2’ in front of the mirror.
Answer:
(i) Converging
(ii)
- Microscope
- Magnifying Glass
- Simple Camera
(iii) Image obtained by a concave mirror when the object is placed at a distance of f/2 is:
- virtual and erect
- larger than the object (magnified); and
- behind the mirror.
![]()
Question 23.
Give reasons for the following:
(i) There is either a convergence or a divergence of magnetic field lines near the ends of a current carrying straight solenoid.
(ii) The current carrying solenoid when suspended freely rests along a particular direction.
(iii) The burnt out fuse should be replaced by another fuse of identical rating.
Answer:
(i) One end of the current carrying solenoid acts like a north pole and the other end acts like a south pole. The magnetic field is stronger near the poles, therefore there is either a convergence (at south pole) or divergence (at north pole) of magnetic field lines near the ends of a current carrying straight solenoid.
(ii) One end of the current carrying solenoid acts like a north pole and the other end a south pole. So, if a current carrying solenoid is suspended freely, it will come to rest pointing in the north and south direction (just like a freely suspended bar magnet).
(iii) The use of an electric fuse prevents the electric circuit and the appliance from a possible damage by stopping the flow of unduly high electric current. A fuse wire works because of its lower melting point. If a fuse with larger rating is used in an appliance, the fuse wire shall not melt and hence would fail to serve the required purpose.
Question 24.
(a) With the help of labelled ray diagram show the path followed by a narrow beam of monochromatic light when it passes through a glass prism.
(b) What would happen if this beam is replaced by a narrow beam of white light?
OR
(a) A person is suffering from both myopia and hypermetropia.
(i) What kind of lenses can correct this defect?
(ii) How are these lenses prepared?
(b) A person needs a lens of power +3D for correcting his near vision and -3D for correcting his distant vision. Calculate the focal lengths of the lenses required to
correct these defects.
Answer:
(a) PE – Incident ray; ∠i – Angle of incidence
EF – Refracted ray; ∠r – Angle of refraction
FS – Emergent ray; ∠e – Angle of emergence
∠A – Angle of the prism; ∠D – Angle of deviation
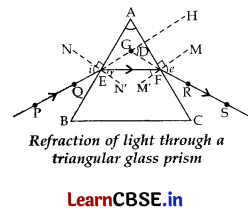
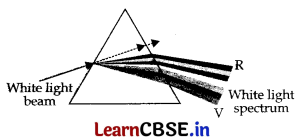
(b) When a narrow beam of while light passes through a prism, it emerges as a spectrum of all components of white light.
OR
(a) (i) When a person is suffering from both myopia and hypermetropia, he should
use spectacles having bifocal lenses.
(ii) Bifocal lenses are prepared in such a manner in which the upper part consists of concave lens (to correct-myopia) used for distant vision and the lower part consists of a convex lens (to correct hypermetropia) used for reading purposes.
(b) When P
1
= +3D: We know, f
1
= \(\frac{1}{\mathrm{P}_1}\) + \(\frac { 1 }{ 3 }\)m = + \(\frac { 100 }{ 3 }\)cm = + 33.3 cm
When P
2
= -3D: As we know, f
2
= \(\frac{1}{\mathrm{P}_2}\) – \(\frac { 1 }{ 3 }\)m = – \(\frac { 100 }{ 3 }\)cm = – 33.3 cm
Section – C
Question 25.
Write balanced chemical equations to explain what happens, when
(i) Mercuric oxide is heated.
(ii) Mixture of cuprous oxide and cuprous sulphide is heated.
(iii) Aluminium is reacted with manganese dioxide.
(iv) Ferric oxide is reduced with aluminium.
(v) Zinc carbonate undergoes calcination.
OR
(i) By the transfer of electrons, illustrate the formation of bond in magnesium chloride and identify the ions present in this compound.
(ii) Ionic compounds are solids. Give reasons.
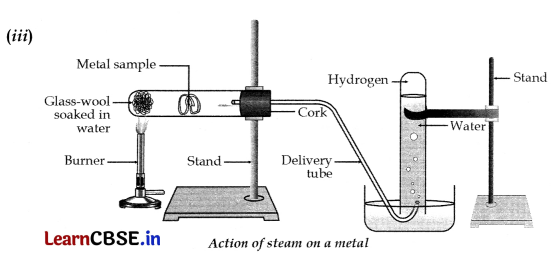
(iii) With the help of a labelled diagram show the experimental set up of action of steam on a metal.
Answer:
(i) When mercuric oxide is heated to about 300° C, it decomposes (gets reduced) to form mercury metal.

(ii) When mixture of cuprous oxide and cuprous sulphide is heated in the absence of air, copper metal and sulphur dioxide gas is liberated.
![]()
(iii) When manganese dioxide is heated with aluminium powder, then manganese metal is produced.
![]()
(iv) Aluminium reduces Ferric oxide to produce iron metal with the evolution of lot of heat. Due to this heat, iron metal is produced in the molten state.
![]()
(v) When zinc carbonate is heated strongly in the absence of air (calcinated), it decomposes to zinc oxide and carbon dioxide.
![]()
OR
(i) Mg has 2 electrons in its outermost shell so it loses its 2 electrons to achieve the inert gas configuration of eight valence electrons and forms positively charged ion or divalent cation.
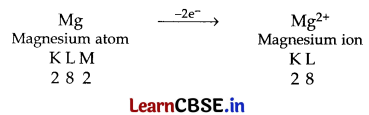
Cl has 7 electrons in its outermost shell so it gains one electron to achieve the stable inert gas configuration and forms negatively charged ion or monovalent anion.
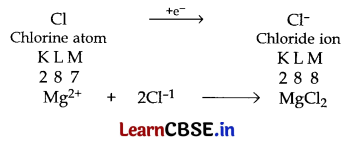
(ii) Ionic compounds are solids because their oppositely charged ions attract one another strongly and form a regular crystal structure.
Question 26.
(a) Compare soaps and detergents on the basis of their composition and cleansing action in hard water.
(b) What happens when ethanol is treated with sodium metal? State the behaviour of ethanol in this reaction.
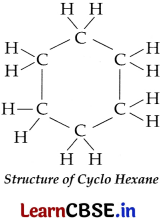
(c) Draw the structure of cyclohexane.
(d) Name the following compound:
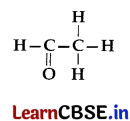
Answer:
(a) Difference between soaps and detergents:
| Soaps | Detergents |
| (i) Soaps are the sodium or potassium salts of long chain carboxylic acids. The ionic group in soaps is: COO – Na + |
(i) Detergents are the sodium salts of long chain benzene sulphonic acids or long chain alkyl sulphates.
The ionic group in a detergent is: -SO 3 – Na + or -SO4 – Na + |
| (ii) Soaps are biodegradable. | (ii) Some detergents are not biodegradable. |
| (iii) Soaps have relatively weak cleansing action. | (iii) Detergents have a strong cleansing action. |
| (iv) Soaps are effective cleanser in soft water. | (iv) Detergents are effective cleansers in both hard and soft water. |
(b) Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas.
![]()
This shows that ethanol does not produce H
+
ion so it is neither acidic nor basic in nature. It is a neutral compound.
(c) Structure of cyclohexane: A saturated cyclic compound in which the carbon atoms are arranged in a ring is a Cyclo Hexane.
(d) CH
3
CHO (ethanal)
Question 27.
(a) Write the correct sequence of steps followed during journey of oxygen rich blood from lungs to various organs of human body.
(b) What happens when the system of blood vessels develops a leak?
Answer:
(a) When the muscles of all the four chambers of the heart are relaxed, the pulmonary vein brings the oxygenated blood (oxygen-carrying blood) from the lungs into the left atrium of the heart.
When the left atrium contracts, the oxygenated blood is pushed into die left ventricle through the valve v
1
.
When the left ventricle contracts, the oxygenated blood is forced into the main artery called ‘aorta’.
The main artery carries blood to all organs (or parts) of the body like head, chest, arms, stomach, intestines, liver, kidneys, trunk and legs (except the lungs). When the oxygenated blood passes through the capillaries of the body organs, then it gives oxygen to the body cells.
(b) When the system of blood vessels develops a leak, it would lead to loss of blood and the loss of blood reduces the pressure which would reduce the efficiency of the pumping system. To avoid this, the blood has platelet cells which circulate around the body and plug these leaks by helping to clot the blood at these points of injury.
Question 28.
(a) Draw a diagram showing germination of pollen on stigma of a flower and mark on it the following organs/parts:
(i) Pollen grain
(ii) Pollen tube
(iii) Stigma
(iv) Female germ cell
(b) State the significance of pollen tube.
(c) Name the parts of flower that develop after fertilization into
(i) Seed
(i) Fruit
OR
(a) “Use of a condom is beneficial for both the sexes involved in a sexual act.” Justify this statement giving two reasons.
(b) Flow do oral contraceptives help in avoiding pregnancies?
(c) What is sex selective abortion? How does it affect a healthy society? (State any one consequence)
Answer:
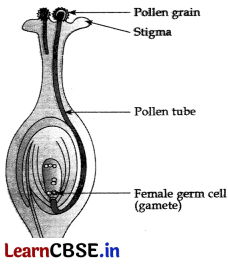
(b) When the pollen lands on the stigma of its own species, it has to reach the female germ cells which are in the ovary. For this, the pollen tube grows out of the pollen grains and travels through the style to reach the ovary.
(c) (i) The ovule develops a tough coat and is gradually converted into a seed.
(ii) The ovary grows and ripens to form a fruit
OR
(a) (i) Condoms are used to prevent the unwanted pregnancies during sexual activity,
(ii) Condoms are used to prevent the spreading of sexually transmitted diseases (STDs) like syphilis, AIDS, etc. Thus use of condoms is beneficial for both the sexes involved in a sexual act.
(b) The oral pills contain hormones which stop the ovaries from releasing ovum into fallopian tube and therefore fertilization cannot occur and unwanted pregnancies can be avoided during sexual activities.
(c) Sex selective abortion is the practice of transmitting a pregnancy based on She sex of the unborn child. The killing of the unborn female child is called female foeticide. Female foeticide is reducing the number of girls drastically in some societies of our country. For a healthy society, the male-female sex ratio must be maintained at almost the same level Due to reckless female foeticide, the male-female child sex ratio is declining at an alarming rate in some sections of our country.
Question 29.
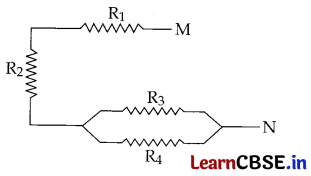
(a) For the combination of resistors shown in the following figure, find the equivalent resistance between M & N.
(b) State Joule’s law of heating.
(c) Why do we need a 5 A fuse for an electric iron which consumes 1 kW power at 220 V?
(d) Why is it impracticable to connect an electric bulb and an electric heater in series.
Answer:
(a) \(\frac{1}{\mathrm{R}^{\prime}}\) = \(\frac{1}{\mathrm{R}_3}\) + \(\frac{1}{\mathrm{R}_4}\) = \(\frac{R_4+R_3}{R_3 R_4}\) … [ ∵ R
3
and R
4
are in parallel combination
∴ R’ = \(\frac{R_3 R_4}{R_3+R_4}\)
Resultant ‘R’ (between M and N) = R
1
+ R
2
+ R’ ….[All three are in series combination
∴ R = R
1
+ R
2
+ \(\frac{R_3 R_4}{R_3+R_4}\)
(b) Joule’s law of heating states that the heat produced in a wire containing current is directly proportional to:
(i) square of current (I
2
)
(ii) Resistance of wire (R)
(iii) time (t) for which current is passed.
∴ H = I
2
Rt
(c) Given: P = 1 kW = 1,000 W;
V = 220 volts;
I = ?
∴ I = \(\frac { P }{ V }\) = \(\frac { 1,000 }{ 220 }\) = 4.54 A
Since the electric iron draws approximately 4.54 A current therefore 5A fuse should be needed.
(d) It is impracticable to connect an electric bulb and an electric heater in series because they need currents of widely different values to operate properly. In a series connection, current remains the same and voltage is divided according to the resistance of the appliance. When we put the electric bulb and the electric heater in series then it will divide the voltage which ultimately divides the power double.
![]()
Question 30.
(a) A security mirror used in a big showroom has radius of curvature 5 m. If. a customer is standing at a distance of 20 m from the cash counter, find the position, nature and size of the image formed in the security mirror.
(b) Neha visited a dentist in his clinic. She observed that the dentist was holding an instrument fitted with a mirror. State the nature of this mirror and reason for its use in the instrument used by the dentist.
OR
Rishi went to a palmist to show his palm. The palmist used a special lens for this purpose.
(i) State the nature of the lens and reason for its use.
(ii) Where should the palmist place/hold the lens so as to have a real and magnified image of an object?
(iii) If the focal length of this lens is 10 cm and the lens is held at a distance of 5 cm from the palm, use lens formula to find the position and size of the image.
Answer:
(a) Here, Security mirror = Convex mirror
Given: Radius of curvature, R = 5 m
∴ Focal length, f = \(\frac { R }{ 2 }\) = \(\frac { 5 }{ 2 }\)m
u = -20 m;
v = ?
Size = h
2
= ?
Nature = ?
According to mirror formula,
\(\frac { 1 }{ v }\) + \(\frac { 1 }{ u }\) = \(\frac { 1 }{ f }\)
⇒ \(\frac { 1 }{ v }\) + \(\frac { 1 }{ -20 }\) = \(\frac { 2 }{ 5 }\)
\(\frac { 1 }{ v }\) = \(\frac { 2 }{ 5 }\) + \(\frac { 1 }{ 20 }\) = \(\frac { 8+1 }{ 20 }\) = \(\frac { 9 }{ 20 }\)
∴ v = \(\frac { 20 }{ 9 }\) = 2.22 m
⇒ \(\frac { -v }{ u }\) = \(\frac { -20 }{ 9 }\) × –\(\frac { 1 }{ 20 }\) = \(\frac { 1 }{ 9 }\)
∴ Image formed is \(\frac { 1 }{ 9 }\) times the object.
∴ Image is virtual (as v is +ve) and diminished.
(b) A dentist uses a concave mirror to see the large images of the teeth of patients because when a tooth is within the focus of a concave mirror, then an enlarged image of the tooth is seen in the concave mirror. Therefore, it becomes easier to locate the defect in the tooth.
OR
(i) A convex lens of smaller focal length is used as a magnifying glass. It will produce , large magnification and hence the object will appear much bigger when seen through it.
(ii) The palmist should hold the lens in such a way that palm is between F and 2F points of the lens, then the palm seen through the convex lens appears much bigger in size than it actually is. The image will be real.
(iii) Given: Focal length of the convex lens, f = +10 cm
u = -5 cm;
v = ?
As we know, = \(\frac { 1 }{ v }\) – \(\frac { 1 }{ u }\) = \(\frac { 1 }{ f }\) (lens formula)
\(\frac { 1 }{ v }\) – \(\frac { 1 }{ (-5) }\) = \(\frac { 1 }{ 10 }\)
⇒ \(\frac { 1 }{ v }\) + \(\frac { 1 }{ 5 }\) = \(\frac { 1 }{ 10 }\)
⇒ \(\frac { 1 }{ v }\) = \(\frac { 1 }{ 10 }\) – \(\frac { 1 }{ 5 }\)
\(\frac { 1 }{ v }\) – \(\frac { 1-2 }{ 10 }\) = \(\frac { -1 }{ 10 }\)
⇒ v = -10 cm
So, the image is formed at 10 cm from the lens on the same side where the object is.
Now, m = \(\frac { v }{ u }\) = \(\frac { -10 }{ -5 }\) = 2
Therefore, image is 2 times (twice) the object.
SET – II Code No. 31/3/2
Except for the following questions, all the remaining questions have been asked in Set I.
Section – B
Question 17.
Complete the following equations:
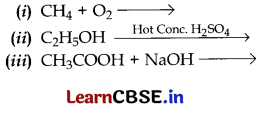
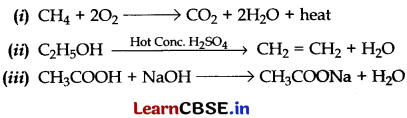
Answer:
Question 19.
(a) Define ecosystem.
(b) Autotrophs are at the first level of food chain. Give reason.
(c) In a food chain of frogs, grass, insects and snakes assign trophic level to frogs. To which category of consumers do they belong?
OR
(a) Explain the role of UV radiation in producing ozone layer.
(b) Mention the reaction involved.
(c) Why is excessive use of CFCs a cause of concern?
Answer:
(a) An ecosystem is a self-contained unit of living things (plants, animals and decomposers) and their non-livmg environment (soil, air and water). An ecosystem needs only the input of sunlight energy for its functioning.
(b) All the food chains begin with an autotroph (green plants) because these are the original source of all food. Thus they are also called the producers in a food chain.
(c) Grass → Insects → Frogs → Snakes
Frogs belong to third trophic level. They belong to carnivores.
OR
(a) & (b) The high energy ultraviolet radiations (UV radiations) coming from the Sun split oxygen gas into free oxygen atoms.
![]()
The free oxygen atoms thus produced are very reactive. One oxygen atom reacts with an oxygen molecule to form ozone molecule.
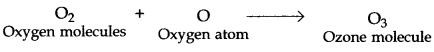
Oxygen molecules Oxygen atom Ozone molecule
(c) CFCs are the chemicals which are widely used in refrigeration as a coolant, when ‘released into the air they react with ozone gas present in the ozone layer and destroy it gradually. Due to this, the ozone layer in the upper atmosphere becomes thinner, allowing more UV rays to pass through it to the earth. These UV rays cause skin cancer and other ailments in humans and animals and also damage the plants. In the absence of the ozone layer, all the life on the earth would be destroyed gradually. Thus, the production of CFCs should be stopped.
Question 20.
Describe an activity to illustrate the phenomenon of phototropism and explain why does this occur.
Answer:
Experiment. We take a potted plant growing in a transparent glass jar. When this potted plant is kept in the open space, the stem of the plant grows straight up towards the Sim while root of the plant grows straight but in the downward direction.
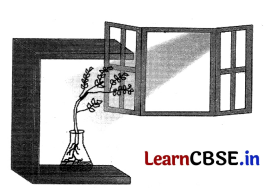
Now keep this plant near the window in a dark room in such a way that sunlight falls on it from the right side only. After some days, we observe that the stem of the plant bends towards the right side towards the light whereas the root bends to the left side away from the light. This experimental setup demonstrates phototropism, i.e., the shoot of the plant bends towards light. This occurs because the plant stem responds to light and bends towards it due to the action of auxin hormone.
Question 22.
(a) Water has refractive index 1.33 and alcohol has refractive index 1.36. Which of the two mediums is optically denser? Give reason for your answer.
(b) Draw a ray diagram to show the path of a ray of light passing obliquely from water to alcohol.
(c) State the relationship between angle of incidence and angle of refraction in the above case.
Answer:
(a) Alcohol having a higher refracting index than water is optically denser than water because higher the refractive index of a substance, more it will change the direction of a beam of light passing through it.
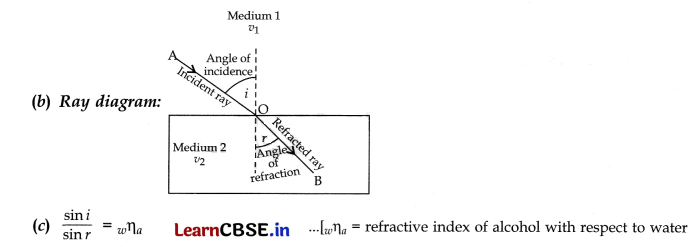
Section – C
Question 26.
(a) Carry out following conversions:
(i) Ethanol to Ethene
(ii) Ethanol to Ethanoic acid
(b) Differentiate between addition reaction and substitution reaction. Give one example of each.
Answer:
(a) (i) Ethanol to Ethene. When ethanol is heated with excess of concentrated sulphuric acid at 443 k, it gets dehydrated to form ethene.
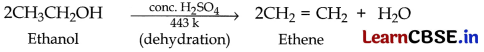
In this reaction conc. sulphuric acid acts as a dehydrating agent.
(ii) Ethanol to ethanoic acid. When ethanol is heated with alkaline potassium permanganate solution (or acidified potassium dichromate solution), it gets oxidised to ethanoic acid.

(b)
| Addition reaction | Substitution reaction |
| The reaction in which an unsaturated hydrocarbon combines with another substance to give a single product is called an addition reaction. | The reaction in which one (or more) hydrogen atoms of a hydrocarbon are replaced by some other atoms is called a substitution reaction. |
| Addition reactions are given by all the alkenes and alkynes. | Substitution reaction is given by saturated hydrocarbons or alkanes. |
|
Example:
Addition of hydrogen occurs when ethene is heated with hydrogen in the presence of nickel catalyst.
|
Methane reacts with chlorine in the presence of sunlight, then one atom of H of methane has been substituted by a ‘Cl’ atom and CH
3
Cl is formed.
|
Question 27.
(a) How do leaves of plants help in excretion? Explain briefly.
(b) Describe the structure and function of a nephron.
Answer:
(a) The gaseous wastes of respiration and photosynthesis in plants (carbon dioxide, water vapours and oxygen) are removed through the stomata in leaves and released into the air. The plants also store some of the waste products in their leaves and plants get rid of these wastes by shedding of leaves also.
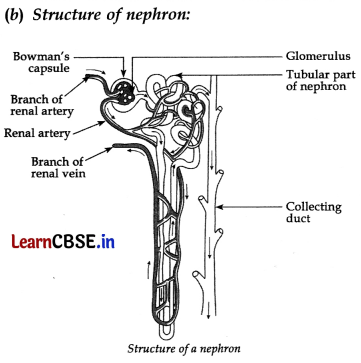
Basic functions of Nephrons:
(i) Filteration. Filteration of the blood takes place in Bowman’s capsule in the capillaries of the glomerulus. Then this filterate passes into the tubular part of the nephron. The filterate contains glucose, amino acids, urea and uric acid and a large amount of water.
(ii) Reabsorption. The filterate flows along the tubule and useful substances such as glucose, amino acids, salts and some water are re-absorbed into the blood by the capillaries surrounding the nephron tubule.
(iii) Urine. The filterate which remains after the re-absorption is called the urine, which is collected from nephron by the collecting duct to carry it to the urinary bladder and then to the urethra.
Question 29.
(a) Define Power and state its SI unit.
(b) A torch bulb is rated 5 V and 500 mA. Calculate its –
(i) Power
(ii) Resistance
(iii) Energy consumed when it is lighted for 2 1/2 hours.
Answer:
(a) Electric power is the electrical work done per unit time.
∴ Power = \(\frac { Work done }{ Time taken }\) = \(\frac { Electrical energy consumed }{ Time taken }\)
SI unit of power is Watt (W). The power of 1 watt is a rate of working of 1 joule per second, i.e.,
1 watt = \(\frac { 1 joule }{ 1 second }\)
(b) Given. Potential difference, V = 5 volts; I = 500 mA = \(\frac { 500 }{ 1000 }\) A = 0.5A
t = 2 1/2 hrs. = \(\frac { 5 }{ 2 }\) × 60 × 60 = 9,000 s
To find; P = ?, R = ?, E = ?
(i) Power, P = V × I = 5 × 0.5 = 2.5 watt
(ii) Resistance, R = \(\frac { V }{ I }\) = \(\frac { 5 }{ 0.5 }\) = \(\frac{5 \times 10}{5}\) = 10 Ω
(iii) Energy consumed, E = P × t = \(\frac { 25 }{ 10 }\) × 9,000 = 22,500 J or 2.25 × 10
4
J
SET – III Code No. 31/3/3
Except for the following questions, all the remaining questions have been asked in Set I & Set II.
Section – B
Question 15.
Identify the type of each of the following reactions.
Also, write balanced chemical equation for each reaction.
(i) A reaction in which the reaction mixture becomes warm.
(ii) A reaction in which an insoluble substance is formed.
Answer:
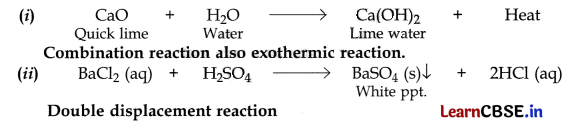
Question 18.
(a) State with reason the consequence of decrease in number of carnivores in an ecosystem.
(b) In a food chain, state the trophic level at which the concentration of harmful chemicals is maximum. Why is it so?
OR
How is ozone layer formed? State its importance to all life forms on earth? Why did the amount of ozone in the atmosphere drop sharply in the 1980s?
Answer:
(a) If the number of carnivores in an ecosystem is decreased then there will be no predator control over the herbivores. Thus the population of herbivores will increase greatly. This will lead to excessive grazing of grass. As a result the density of producers will be very much reduced and the lush-green forest will turn into a desert area having no vegetation at all.
(b) Some harmful chemicals like pesticides, when absorbed by the plants through soil and water, get transferred from first trophic level to the last trophic level of the food chain. As these chemicals are non-degradable, their concentration in the bodies of living organisms at each trophic level progressively increases. This increase in the concentration of harmful chemicals in the body of living organisms at each trophic level of a food chain is called biological magnification. The level of concentration of chemicals is maximum for human beings as they are at the highest trophic level.
OR
Forination of ozone layer: The high energy ultraviolet radiation (UV radiation) coming from the Sun splits oxygen gas into free oxygen atoms
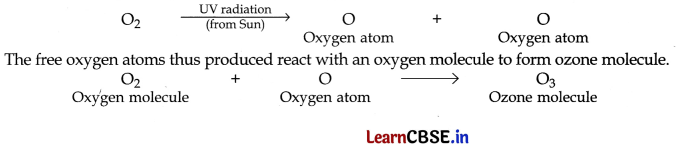
Importance of ozone: Ozone layer is very important for the existence of life on earth. The function of the ozone layer in the upper atmosphere is to absorb most of the harmful ultraviolet radiations coming from the Sun and prevent them from reaching the earth’s surface.
Depletion of ozone: Chlorofluorocarbons or the CFCs are the main cause for the depletion of ozone layer which was first identified during 1980s (1985). The ozone layer depleted due to over use of CFCs that are released by soaps solvents, spray aerosols, refrigerators and air conditioners.
![]()
Question 22.
(a) State Snell’s law of refraction of light.
(b) When a ray of light travelling in air enters obliquely into a glass slab, it is observed that the light ray emerges parallel to the incident ray but it is shifted sideways slightly. Draw a labelled ray diagram to illustrate it.
Answer:
(a) Laws of refraction of
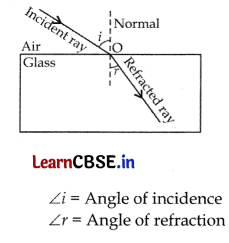
(i) First law. The incident ray, the refracted ray and the normal at the point of incidence, all lie in the same plane.
(ii) Second law. The ratio of sine of angle of incidence to the sine of angle of refraction is constant for a given pair of media. The second law of refraction is called Snell’s law.
Mathematically, it can be written as:
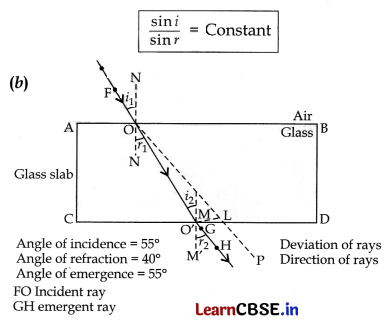
Section – C
Question 26.
A metal ‘M’ is stored under kerosene. It vigorously catches fire if a small piece of this metal is kept open in air. Dissolution of this metal in water releases great amount of energy and the metal catches fire. The solution so formed turns red litmus blue.
(a) Name the metal ‘M’.
(b) Write formula of the compound formed when this metal is exposed to air.
(c) Why is metal ‘M’ stored under kerosene?
(d) If oxide of this metal is treated with hydrochloric acid, what would be the products?
(e) Write balanced equations for:
(i) Reaction of ‘M’ with air.
(ii) Reaction of ‘M’ with water.
(iii) Reaction of metal oxide with hydrochloric acid.
OR
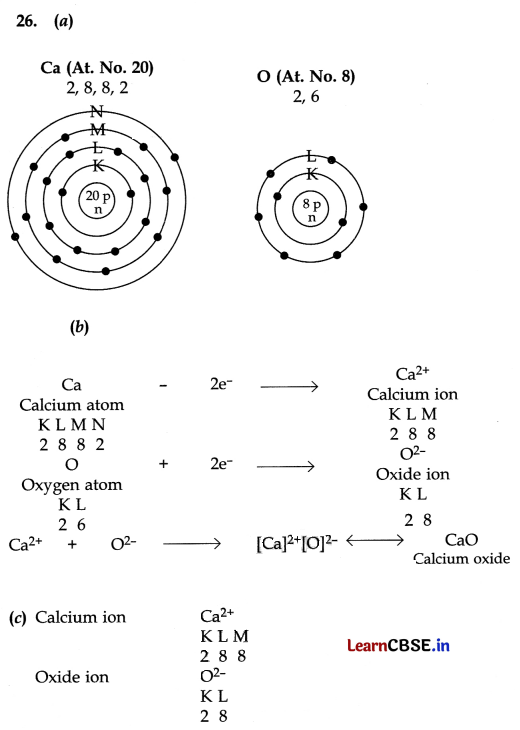
(a) Write electron dot structures of Ca (At. No. 20) and O (At. No. 8).
(b) Show the formation of calcium oxide by transfer of electrons.
(c) Name the ions present in this compound.
(d) List four important characteristics of this compound.
Answer:
(a) Metal ‘M’ is sodium.
(b) When sodium metal is exposed to air, it reacts with oxygen of air to form basic oxide called sodium oxide (Na
2
O).
(c) Sodium metal is very reactive, it reacts vigorously with oxygen of air and catches fire and starts burning when kept open in air. Therefore it is stored under kerosene oil to prevent its reaction with the oxygen, moisture and carbon dioxide of air.
(d) When sodium oxide is treated with HCl, it produces salt (NaCl) and water.
(e)
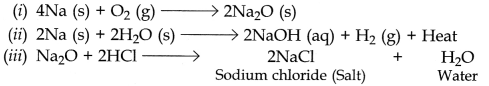
OR
(d) Characteristics of ionic compound:
(i) Ionic compounds are generally hard and solids.
(ii) Ionic compounds have high melting and boiling points.
(iii) Ionic compounds are mostly soluble in water and insoluble in solvents like kerosene oil, alcohols, etc.
(iv) In their molten state or aqueous solution these are good conductors of electric current.
Question 28.
(a) Suggest any two categories of contraceptive methods to control the size of human population which is essential for the prosperity of a country. Also explain about each method briefly.
(b) Name two bacterial and two viral infections each that can get sexually transmitted.
(c) List two advantages of using condom during sexual act.
OR
(a) Draw a diagram to show spore formation in Rhizopus.
(b) With the help of an example differentiate between the process of Budding and Fragmentation.
(c) Why is vegetative propagation practiced for growing some type of plants?
Answer:
(a) There are three different methods of contraception:
(i) Barrier methods. In these methods, physical devices such as condoms, diaphragms and cervical caps are used. These devices prevent the entry of sperm in the female genital tract, thus acting as a barrier between them.
(ii) Surgical methods. There are surgeries that can be carried out in males and females. In males, a small portion of the sperm duct (vas deferens) is blocked by a surgical operation. This prevents the sperms from coming out.
In females, a small portion of the fallopian tubes (oviducts) is blocked by a surgical operation. It prevents the egg from reaching the uterus. In both the cases, fertilisation will not take place.
(iii) Chemical methods. This category of contraceptives acts by changing the hormonal balance of the body so that eggs are not released and fertilisation cannot occur. Females use two types of pills for preventing pregnancies, i.e., > oral pills and vaginal pills. The oral pills contain hormones which stop the ovaries from releasing ovum into the fallopian tube. This is also called oral contraceptives (OC).
Other contraceptive devices such as loop or the copper-T are placed in the uterus to prevent pregnancy.
(b) (i) Sexually transmitted bacterial infections: Gonorrhoea and Syphilis
(ii) Sexually transmitted viral infections: AIDS and Warts
(c) Advantages of using condom during sexual act:
(i) Use of condoms prevents the spreading of sexually transmitted diseases like AIDS, Syphilis etc.
(ii) Use of condom controls human population by checking the unwanted pregnancies during sexual acts.
OR
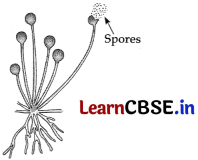
(a) Spore formation in Rhizopus:
(b) Difference between the process of Budding and Fragmentation:
| Budding | Fragmentation |
| On budding, a small part of the body of the parent organism grows out as a bud which then detaches and becomes a new organism. | In fragmentation, the body of a simple multicellular organism breaks into two or more pieces on maturing, each of which subsequently grows to form a complete new organism. |
| Budding is observed in simple multicellular organisms like hydra and unicellular organisms like yeast. | The breaking up of the body of an organism to form new organisms occurs naturally when the parent organism matures. |
| Hydra and yeast reproduce by this method which is an asexual type of reproduction. | The simple multicellular organisms like spirogyra and sea anemones can reproduce by this method. |
Example of Budding :
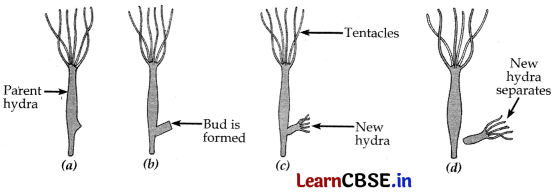
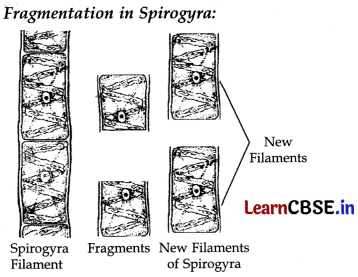
(c) Vegetative propagation practiced for growing some type of plants because-
(i) the fruit trees grown through vegetative propagation bear fruits much earlier.
(ii) banana and grapes produce either very few seeds or do not produce viable seeds. Therefore their plants are grown by vegetative propagation.
Question 30.
(a) An electric bulb is rated at 200 V; 100 W. What is its resistance?
(b) Calculate the energy consumed by 3 such bulbs if they glow continuously for 10 hours for the complete month of November.
(c) Calculate the total cost if the rate is ₹6.50 per unit.
Or
(a) What is meant by the statement, “The resistance of a conductor is one ohm”?
(b) Define electric power. Write an expression relating electric power, potential difference and resistance.
(c) How many 132 Ω resistors in parallel are required to carry 5 A on a 220 V line?
Answer:
(a) Electric bulb potential difference = 200 V;
P = 100 W;
R = ?
As we know, P = VI
∴ I = \(\frac { P }{ V }\) = \(\frac { 100 }{ 200 }\) = 0.5 A
Now, V = IR
∴ R = \(\frac { V }{ I }\) = \(\frac { 200 }{ 0.5 }\) = \(\frac{200 \times 10}{5}\) = 400 Ω
(b) Given: No. of bulbs = 3
Power = 100 W = 100/1000 kW = 1/10 kW
Time = 10 hrs. × 30 days (in November) = 300 hrs.
∴ Energy consumed, E = 3 × \(\frac { 1 }{ 10 }\) × 300 = 90 kWh = 90 electrical units
(c) Cost of 1 unit = ₹ 6.50
∴ Cost of 90 units = 90 × ₹ 6.50 = ₹ 585
OR
(a) 1 ohm is the resistance of conductor such that when a potential difference of 1 volt is applied to its ends, a current of 1 ampere flows through it.
(b) Electric power is the electrical work done per unit time.
Power (P) = \(\frac { Work done }{ Time taken }\) = \(\frac{V^2}{R}\)
(c) Let number of resistors be n.
Given. R = 132 W;
I = 5 A;
V = 220 V
Resultant R’ for n resistances combined in parallel:
\(\frac{1}{R^{\prime}}\) = \(\frac{1}{R}\) + \(\frac{1}{R}\) + …. n times
⇒ \(\frac{1}{R^{\prime}}\) = \(\frac{n}{R}\)
⇒ R’ = \(\frac{R}{n}\) = \(\frac{132}{n}\) Ω
Now, V = IR’
⇒ 220 = 5 × \(\frac{132}{n}\) Ω
⇒ n = \(\frac{5 \times 132}{220}\) = 3
∴ No. of resistors = 3