Students can find that CBSE Previous Year Question Papers Class 10 Science with Solutions and CBSE Class 10 Science Question Paper 2018 Comptt (Delhi & Outside Delhi) effectively boost their confidence.
CBSE Class 10 Science Board Question Paper 2018 Comptt (Delhi & Outside Delhi) with Solutions
Time allowed: 3 hours
Maximum marks: 80
students
- The Question Paper comprises of two sections, A and B. You are to attempt both the sections.
- All questions are compulsory.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question numbers 1 to 2 in Section A are one mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are based on practical skills. Each question is a two marks question. These are to be answered in brief.
Section – A
Question 1.
Mendel took tall pea plants and short pea plants and produced F
1
, progeny through cross-fertilisation. What did Mendel observe in the F
1
progeny?
Answer:
Mendel observed that all pea plants were tall in F
1
progeny. This meant that only one of the parental traits was seen.
Question 2.
Define power of the lens. Write its S.I. unit.
Answer:
Power of lens. The reciprocal of focal length of a lens is known as its power, the focal length is expressed in metres.
S.I. unit of power of lens is Dioptre (D).
![]()
Question 3.
Carbon has four electrons in its valence shell. Which type of compounds can be formed by carbon atom and why? Give any one example of such compounds.
Answer:
Carbon has 4 electrons in its valence shell, so it should either lose 4 electrons or gain 4 electrons to achieve the inert gas configuration. But it is not possible to remove 4-electrons from a carbon atom. Also it is not possible to add as many as 4-electrons to the carbon atom due to high energy requirement. Therefore carbon atom achieves the inert gas configuration only by sharing of electrons. Thus carbon always forms covalent bonds.
Question 4.
Different parts of brain are associated with specific functions. Name the part of human brain which perform the following functions:
(a) Sensation of feeling full
(b) Vomiting
(c) Picking up a pencil
(d) Riding a bicycle
Answer:
(a) Sensation of feeling full – Cerebrum/Fore brain
(b) Vomiting – Medula/Hind brain
(c) Picking up a pencil – Cerebellum/Hind brain
(d) Riding a bicycle – Cerebellum/Hind brain
Question 5.
An object is kept 40 cm in front of a concave mirror of focal length of 20 cm. Find the position and nature of the image. Draw a ray diagram to show the formation of image in this case.
Answer:
Concave mirror
u = -40 cm;
f = -20 cm;
V = ?
nature = ?
According to mirror formula
\(\frac { 1 }{ v }\) + \(\frac { 1 }{ u }\) = \(\frac { 1 }{ f }\) ⇒ \(\frac { 1 }{ v }\) + \(\frac { 1 }{ -40 }\) = \(\frac { 1 }{ -20 }\)
⇒ \(\frac { 1 }{ v }\) + \(\frac { 1 }{ 40 }\) = \(\frac { -1 }{ 20 }\)
⇒ \(\frac { 1 }{ v }\) + \(\frac { -1 }{ 20 }\) = \(\frac { 1 }{ 40 }\) = \(\frac { -2+1 }{ 40 }\) = \(\frac { -1 }{ 40 }\)
v = -40 cm
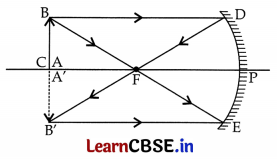
Position: Image is formed 40 cm in front of the concave mirror.
Nature: Image is real and inverted.
Question 6.
In the reaction:
MnO
2
+ 4HCl → MnCl
2
+ 2H
2
O + Cl
2
(a) Name the compound:
(i) oxidised,
(ii) reduced.
(b) Define oxidation and reduction on its basis.
Answer:
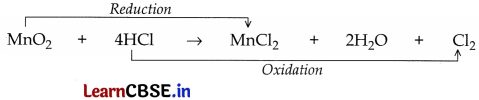
(a) (i) Compound oxidised = HCl;
(ii) Compound reduced = MnO
2
(b) Oxidation. The addition of oxygen to a substance or the removal of hydrogen from a substance is called oxidation.
Reduction. The addition of hydrogen to a substance or the removal of oxygen from a substance is called reduction.
Question 7.
1 g of solid sodium chloride is taken in a clean and dry test tube and 2 mL of cone, sulphuric acid is added to it. If the gas evolved is tested first with dry and then with wet blue litmus paper, in which case will the litmus paper change colour? Give reason for your answer. What inference can be drawn about the nature of the evolved gas? Support your answer with a chemical equation for the reaction.
OR
(a) For the preparation of cakes, baking powder is used. If at home your mother uses baking soda instead of baking powder, how will it affect the taste of the cake and why?
(b) How is baking soda converted into baking powder?
(c) What makes the cake soft and spongy?
Answer:
2NaCl + H
2
SO
4
→ Na
2
SO
4
+ 2HCl
Concentrated sulphuric acid reacts with sodium chloride to form hydrogen chloride gas. When the gas evolved is tested with dry blue litmus paper then there is no change in the colour of the dry blue litmus paper. This shows that HCl gas does not behave as an acid in the absence of water.
When the gas is tested with wet blue litmus paper then the blue litmus paper turns red. This shows that HCl gas shows acidic behaviour in the presence of water.
Explanation:
Dry HCl does not contain any hydrogen ions (H
+
) in it, so it does not show acidic behaviour so HCl gas does not change the colour of the dry blue litmus paper.
When HCl gas is tested with wet blue litmus paper then HCl gas first dissolves in water to form HCl acid solution.
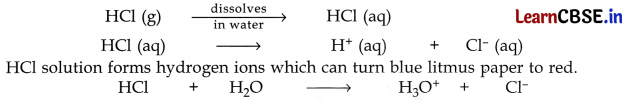
can
For the preparation of cake if only sodium hydrogen carbonate (baking soda) is used instead of baking powder then sodium carbonate is formed from it by the action of heat during baking. This will give a bitter taste to the cake. The advantage of using baking powder is that tartaric acid present in it can react with sodium carbonate formed and neutralise it to sodium tartrate salt and the cake has a pleasant taste.
–
(c) When baking powder mixes with water then sodium hydrogen carbonate reacts with tartaric acid to evolve CO
2
gas
NaHCO
3
(aq) + H
+
(aq) → Na
+
(aq) + CO
2
(g) + H
2
O (l)
The CO
2
gas produced gets trapped in the wet dough and bubbles out slowly causing the cake (or bread) to rise and become soft and spongy.
Question 8.
What happens when hydrogen is added to a vegetable oil in the presence of nickel? Name the reaction and write one difference between the physical property of the vegetable oil and the product obtained in this reaction. Write the role of nickel in this reaction.
Answer:
• Vegetable oils are unsaturated fats having double bonds between some of their carbon atoms. When a vegetable oil is heated with hydrogen in the presence of finely divided nickl then a saturated fat called vegetable ghee is formed. This reaction is called hydrogenation of oils.
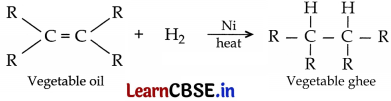
• Vegetable oil is in liquid state at room temperature whereas vegetable ghee (saturated fat) is solid (or semi-solid) at room temperature.
• Nickel acts as a catalyst in the process of hydrogenation of vegetable oil.
Question 9.
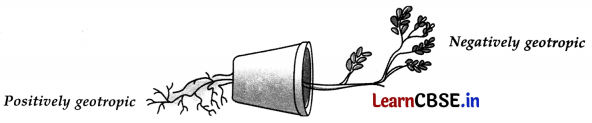
What is geotropism? Draw a labelled diagram of a potted plant showing positive geotropism and negative geotropism.
Answer:
The response of plants to gravity is called geotropism. The roots of plants always grow downwards in response to gravity showing positive geotropism. The shoots of the plants always grow up, away from the gravity showing negative geotropism.
Diagram of a potted plant showing positive geotropism and negative geotropism:
Question 10.
(a) What is meant by the dispersion of white light?
(b) Explain why the planets do not twinkle but the stars twinkle?
Answer:
(a) Dispersion of light. The splitting of white light into its component colours on passing through a prism is called dispersion of light.
(b) The planets are much closer to the earth. A planet can be considered as a collection of large number of point-sized sources of light. So the total variation in the amount of light entering our eye from all the individual point-sized sources will average out to zero thereby nullifying the twinkling effect. On the other hand, stars twinkle because stars are point-sized sources of energy therefore the continuously changing atmosphere causes atmospheric refraction which causes variation in light.
![]()
Question 11.
We wish to obtain an equal sized inverted image of a candle flame on a screen kept at a distance of 4 m from the candle flame.
(a) Name the type of lens that should be used.
(b) What should be the focal length of the lens and at what distance from the candle flame the lens be placed?
(c) Draw a labelled diagram to show the image formation in this case.
OR
A 5 cm tall object is placed at a distance of 30 cm from a convex mirror of focal length 15 cm. Find the position, size and nature of the image formed.
Answer:
(a) Convex lens
(b) Focal length of the lens should be 2 m. Distance of candle flame from the lens is 4 m.
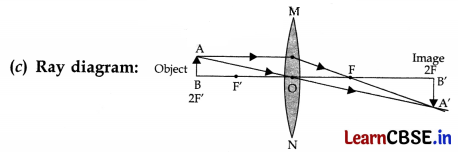
OR
In the given Convex mirror –
Object height, h
1
= +5 cm
Focal length, f = +15 cm
Image height, h
2
= ?
Object distance, u = -30 cm
Image distance, v = ?
Nature = ?
According to mirror formula,
\(\frac { 1 }{ v }\) + \(\frac { 1 }{ u }\) = \(\frac { 1 }{ f }\)
⇒ \(\frac { 1 }{ v }\) + \(\frac { 1 }{ -30 }\) = \(\frac { 1 }{ +15 }\)
\(\frac { 1 }{ v }\) = \(\frac { 1 }{ 15 }\) + \(\frac { 1 }{ 30 }\) = \(\frac { 2+1 }{ 30 }\) = \(\frac { 3 }{ 30 }\) = \(\frac { 1 }{ 10 }\)
∴ v = +10 cm
The image is formed (10 cm) behind the convex mirror. Since the image is formed behind the convex mirror, its nature will be virtual (as v is +ve).
∵ \(\frac{h_2}{h_1}\) = \(\frac{-v}{u}\)
⇒ \(\frac{h_2}{5}\) = \(\frac{-(+10)}{-30}\)
h
2
= \(\frac { 10 }{ 30 }\) × 5
∴ h
2
= \(\frac { 5 }{ 3 }\) = +1.66 cm
Thus size of the image is 1.66 cm and it is erect (as h
2
is +ve).
∴ Nature of image = Virtual and effect
Question 12.
Consider the following circuit:
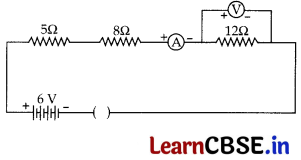
What would be the readings of the ammeter and the voltmeter when key is closed? Give reason to justify your answers.
OR
What is electrical resistivity? Derive its SI unit. In a series electrical circuit comprising a resistor made up of a metallic wire, the ammeter reads 100 mA. If the length of the wire is doubled, how will the current in the circuit change? Justify your answer.
Answer:
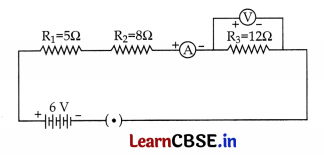
R
1
= 5 Ω;
R
2
= 8 Ω;
R
3
= 12 Ω;
Resultant resistance, R = R
1
+ R
2
+ R
3
(series)
R = 5 + 8 + 12 = 25 Ω; V = 6 V; I = ?
V = IR
I = \(\frac { V }{ R }\) = \(\frac { 6 }{ 25 }\) = 0.24 A
∴ Reading in ammeter is 0.24 A
R
3
= 12 Ω
I
3
= 0.24 A ….. [I
1
= I
2
= I
3
= I
V
3
= ?
⇒ V
3
= I
3
× R
3
= 0.24 × 12 = 2.88 Volts
OR
Electrical resistivity of the material of a conductor is the resistance offered by the conductor of length 1 m and area of cross-section 1 m
2
. Numerically it is equal to the resistance of a rod of that substance which is 1 metre long and 1 square metre in cross-section.
ρ = \(\frac { RA }{ l }\)
since unit of R = ohm;
A = (metre)
2
;
l = (metre)
unit of resistivity, ρ = \(\frac{\text { ohm } \times(\text { metre })^2}{(\text { metre })}\) = ohm-metre (or Ω m)
R = \(\frac { ρl }{ A }\)
If l’ = 2l … [∵ Lenght is doubled
∴ A’ = A
New resistance, R’ = \(\frac{\rho l^{\prime}}{\mathrm{A}^{\prime}}\) = \(\frac{\rho \times 2 l}{\mathrm{~A}}\) = \(\frac{2 \rho l}{\mathrm{~A}}\)
R’ = 2R
∴ Resistance will be doubled since length is doubled.
∵ V = IR …[Ohm’s law ⇒ I = \(\frac { V }{ R }\) ⇒ I’ = \(\frac { V’ }{ R’ }\)
∵ V’ = V; R’ = 2R
∴ I’ = \(\frac { V }{ 2R }\)
⇒ I’ = \(\frac { 1 }{ 2 }\) × I
∵ I = 100 mA
⇒ I’ = \(\frac { 1 }{ 2 }\) × 100 = 50 mA
∴ Current in the circuit is reduced to half.
Question 13.
Calculate the total cost of running the following electrical devices in the month of September, if the rate of 1 unit of electricity is ₹6.00.
(i) Electric heater of 1000 W for 5 hours daily.
(ii) Electric refrigerator of 400 W for 10 hours daily.
Answer:
(i) Energy consumed by electric heater = P × time = 1000 W × 5 h = 5000 Wh
(ii) (Energy consumed by refrigerator = P × time = 400 × 10 = 4000 Wh
Total energy consumed in one day = 5000 Wh + 4000 Wh
= 9000 Wh = \(\frac { 9000 }{ 1000 }\)k Wh = 9 k Wh
Total energy consumed in the month of September = 30 × 9 = 270 kWh = 270 units
Cost of 1 unit of electricity = ₹6
∴ Cost of 270 units of electricity = ₹6 × 270 = ₹1,620
Question 14.
While teaching the chapter “Our Environment” the teacher stressed upon the harmful effects of burning of fossil fuels, plastic, paper etc. The students noticed the extensive use of plastic and polythene in daily life, which can be avoided and the surroundings can be kept clean. They decided to make their school “plastic and polythene” free and motivated each other for its minimum use.
(a) Why should the use of polythene and plastic be reduced in daily life?
(b) In what ways the students would have avoided the use of plastic and polythene in their school?
(c) How the students would have motivated each other for the success of their decision?
Answer:
(a) Polythene and plastic are non-biodegradable materials which cannot be decomposed by micro-organisms. Burning of plastic in open air leads to environmental pollution due to release of poisonous gases. When polythene is thrown on land it makes the soil less fertile. When thrown in water it chokes our water bodies and harms the aquatic life. If eaten by stray animals, the plastic bags can choke their alimentary canal.
(i)
- Stop using polythene bags and start using bags made of cloth or jute.
- One must collect the used and discarded items of plastic and send them to the respective industries for making fresh plastic items.
- The use of disposable items like cold drink cans, stationary items, glasses should be avoided. Have freshly squeezed juice or eat fruit instead of buying juice in plastic bottles. It is healthier too.
- Students should carry tiffin and water in steel containers instead of plastic containers.
- Encourage the use of ink pens instead of ball pens made of plastic.
The
- create a vibrant school board about the improvement of environment;
- Holding a recycling challenge to clean the school and design the recycle bin;
- Students should create and put up signs and posters around the school about the harmful effects of using plastic.;
- Speeches in morning assembly, debate competitions in the favour of environment safety should be conducted;
- Start an Eco or Sustainable Club; and
- Make new recycling and composting collection stations in the school and nearby localities.
![]()
Question 15.
What is “Sustainable Management of Natural Resources”? Why is it necessary? Which one out of reuse and recycle, would you practise in your daily life and why?
Answer:
The development which meets the current basic human needs and also preserves the resources for the needs of future generations is called sustainable management. A system of controlling the use of natural resources in such a way as to avoid their wastage and to use them in the most effective way is called sustainable management.
Advantages:
(i) The resources of the earth are limited. Proper management of these natural resources can ensure that these are used judiciously so that they fulfill the needs of the present generation and also last for the generations to come.
proper
management
of
natural
Question 16.
(a) List in tabular form any three chemical properties on the basis of which metals and non-metals are differentiated.
(b) State two ways to prevent the rusting of iron.
Answer:
| Metals | Non-metals |
| (i) Metals lose electrons to form positive ions; are electropositive in nature. | (i) Non-metals gain electrons to form negative ions; are electronegative in nature. |
| (ii) React with dilute acids to liberate hydrogen gas. | (ii) Do not react with dilute acids. |
(iii) Metals react with oxygen to form basic oxides
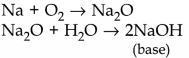
Metals react with chlorine to form ionic chlorides 2Na + Cl 2 → NaCl A few reactive metals react with hydrogen to form ionic metal hydrides. 2Na + H 2 → 2NaH |
(iii) Non-metals react with oxygen to form acidic oxides
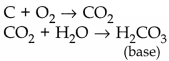
Non-metals react with chlorine to form covalent chlorides H 2 + Cl 2 → 2HCl Non-metals react with hydrogen to form covalent hydrides. H 2 + S → H 2 S |
Question 17.
What is homologous series. Write the properties of compounds of a homologous series.
Or
Write the names of functional groups of alcohols, aldehydes and corboxylic acids. Draw structures of these functional groups also.
Answer:
A series of carbon compounds in which the same functional group substitutes for hydrogen on a carbon chain is called a homologous series. There is a difference of – CH
2
in the molecular formulae of two nearest compounds of a homologous series. Each such series has same general molecular formula and has a general scientific name. There is a difference of 14 u (unified mass) in the molecular masses of two nearest compounds of a series.
Characteristics:
(i) As the molecular mass increases in any homologous series, the melting and boiling points increase, therefore a gradation in physical properties is observed.
(ii) The chemical properties, which are determined by the functional group, remain similar in a homologous series.
Examples of homologous series are alkanes, alcohols, aldehydes etc.
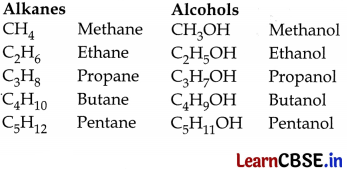
OR
(i) Alcohols:
Name of functional group:
Structure of functional group:
![]()
(ii) Aldehydes:
Name of functional group:
Structure of functional group:
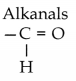
(iii) Carboxylic acids:
Name of functional group:
Structure of functional group:
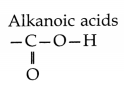
Question 18.
(a) Write the reaction that occurs when glucose breaks down anaerobically in yeast.
(b) Write the mechanism by which fishes breath in water.
(c) Name the balloon like structures present in lungs. List its two functions.
(d) Name the respiratory pigment and write its role in human beings.
OR
(a) Name the process and explain the type of nutrition found in green plants. List the raw materials required for this process. Given chemical equation for the mentioned process.
(b) Write three events that occur during this process.
Answer:
(a) Break down of glucose anaerobically in yeast:
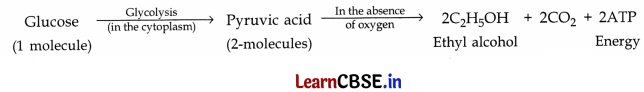
(b) The fishes have special organs of breathing called ‘gills’. The fish breathes by taking in water through its mouth and forces it past the gills, the gills extract dissolved oxygen from this water which is then taken up by the blood. The water then goes out through the gills slits.
(c) The balloon like structures present in lungs are called alveoli.
Two functions:
- The alveoli provide a surface where the exchange of gases can take place.
- The walls of alveoli are covered by extensive network of blood vessels or capillaries in which oxygen from alveoli dissolves and are carried by the blood to the heart for pumping. In the same way, carbon dioxide from the body cells are carried by the blood capillaries to the alveoli from where it goes out of the body.
(d) In human beings, the respiratory pigment is haemoglobin which has a very high affinity for oxygen. This pigment is present in the red blood corpuscles.
OR
(a) Green plants obtain their food by the process of photosynthesis. This mode of nutrition is called autotrophic nutrition. This type of nutrition involves the intake of simple inorganic materials from the environment and use an external energy source like the sun to synthesise complex high energy organic material. The process by which green plants make their own food (glucose) using inorganic material like carbon-dioxide and water by using sunlight energy is called photosynthesis.

(b) Events that occur during photosynthesis:
(i) Absorption of sunlight energy by chlorophyll.
(ii) Conversion of light energy into chemical energy and splitting of water into hydrogen and oxygen by light energy.
(iii) Reduction of carbon dioxide by hydrogen to form carbohydrates like glucose by utilising the chemical energy (obtained by the transformation of light energy).
Question 19.
(a) What is variation? How is variation created in a population? How does the creation of variation in a species promote survival?
(b) Explain how, offspring and parents of organisms reproducing sexually have the same number of chromosomes.
Answer:
(a)• The difference in the characters among individuals of a species is called variation.
resources
takes
into
Question 20.
(a) What is presbyopia? State its cause. How is it corrected?
(b) State the functions of the following in human eye: 5
(i) Iris, (ii) Pupil
Answer:
(a) Presbyopia is a defect of vision due to which an old person cannot see nearby as well as far off objects clearly due to loss of power of accommodation of the eye. Causes: Presbyopia occurs in old age due to gradual weakening of the ciliary muscles and diminishing flexibility of the eye-lens. The near point of the old person having presbyopia gradually recedes and becomes much more than 25 cm away. Correction: Presbyopia defect is corrected by using spectacles having bi-focal lens.
(b) Function of iris in human eye. Iris regulates the amount of light entering the eye by adjusting the size of the pupil.
Function of pupil in human eye. The amount of light entering the eye can be controlled by the pupil. If the intensity of outside light is low, then the pupil expands to allow more light to enter the eye. On the other hand, if outside intensity of light is high, then the pupil contracts so that less light enters the eye.
Question 21.
(a) What are magnetic field lines? How is the direction of magnetic field at a point in a magnetic field determined using field lines?
(b) Why do two magnetic field lines never cross each other?
(c) Where is the magnetic field strength maximum in the magnetic field of a bar magnet?
Answer:
(a) Magnetic field lines are the lines drawn in a magnetic field along which a north magnetic pole would move. Magnetic field lines are also known as magnetic lines of force. Direction of magnetic field lines is determined by the following activity.
A bar magnet is placed on a sheet of paper and its boundary is marked with a pencil. A magnetic compass is brought near the N-pole of the bar magnet. It is observed that N-pole of magnet repels the N-pole of compass needle due to which the tip of the compass needle moves away from the N-pole.
Thus a magnetic field pattern is obtained around a bar magnet. Each magnetic field line is directed from the north pole of a magnet to its south pole. The field lines are closest together at the two poles of the bar magnet. The strength of magnetic field is indicated by the degree of closeness of the field lines. So the magnetic field is the strongest near the poles.
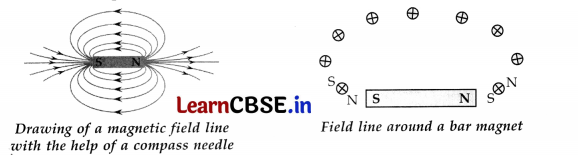
(b) If two field lines cross each other at any point then at that point, direction of magnetic field will be in two directions which is not possible. Field direction at any point is only in one direction.
(c) Magnetic field strength is maximum near the poles of the bar magnet. Near the poles field lines are very closely packed.
Section – B
Question 22.
In the laboratory of a school, the sample of hard water is not available “to study the comparative cleaning capacity of a sample of soap in soft and hard water”. Which salt from the laboratory can be added to tap water to make it hard? In the experiment how is cleaning capacity of soap compared?
Answer:
Calcium and Magnesium salts (like CaCl
2
, MgCl
2
) are added to the tap water to make it hard.
• Take 10 ml of soft water (tap water) in a test tube and 10 ml of hard water (just prepared above) in a separate test tube. Add few drops of soap solution in both the test tubes. It is observed that, the test tube containing soft water produces large amount of foam but the test tube with hard water will have white and curdy appearance of precipitate.
consideration
long-term
![]()
Question 23.
In a school laboratory the students are studying the properties of ethanoic acid through certain experiments. How can they test its acidic nature? Give two tests.
Answer:
- Ethanoic acid turns blue litmus solution to red.
- Ethanoic acid gives orange colour with universal indicator.
- Ethanoic acid remains colourless in phenolphthalein.
- On adding solid sodium carbonate or sodium hydrogen carbonate, brisk effer-vescence is observed.
Question 24.
Draw a labelled diagram of the experimental set up for the study of liberation of carbon dioxide gas during respiration.
Answer:
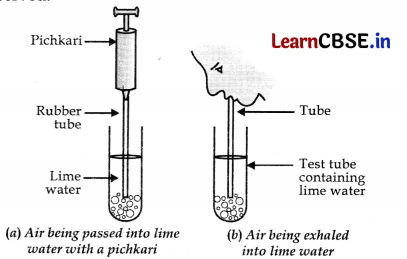
Question 25.
Draw diagrams showing reproduction in yeast in proper sequence.
Answer:
This process is known as Budding method of Asexual Reproduction. Diagram:
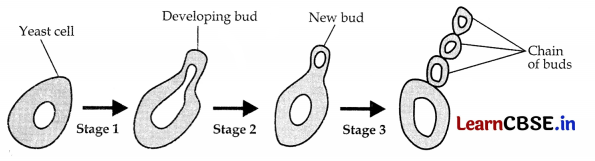
Question 26.
While tracing the path of a ray of light passing through a rectangular glass slab a student tabulated his observations. If in his experiment for two readings he takes two values of ∠i as 40° and 50°, what should be the correct values of ∠e and ∠r in each case?
OR
Draw a labelled ray diagram for the formation of image by a convex lens of focal length 15 cm when the object is placed at a distance of 25 cm from the lens. Determine the size of the image formed, if size of the object is 4 cm.
Answer:
Case I.
When ∠i = 40°
then ∠e = 40°
∠r < 40° or ∠r = 23°
∵ ∠i = ∠e
\(\frac { sin (i) }{ sin (r) }\) = Constant
Case II:
When ∠i = 50°
∠e = 50°
∠r < 50° or ∠r = 30°
OR
Convex lens
f = +15 cm;
u = -25 cm;
h
1
= 4 cm
\(\frac { 1 }{ v }\) – \(\frac { 1 }{ u }\) = \(\frac { 1 }{ f }\)
⇒ \(\frac { 1 }{ v }\) – \(\frac { 1 }{ -25 }\) = \(\frac { 1 }{ 15 }\)
\(\frac { 1 }{ v }\) + \(\frac { 1 }{ 25 }\) = \(\frac { 1 }{ 15 }\)
⇒ \(\frac { 1 }{ v }\) = \(\frac { 1 }{ 15 }\) – \(\frac { 1 }{ 25 }\) = \(\frac { 5-3 }{ 75 }\) = \(\frac { 2 }{ 75 }\)
v = \(\frac { 75 }{ 2 }\) = 37.5 cm
\(\frac{h_2}{h_1}\) = \(\frac{v}{u}\)
⇒ \(\frac{h_2}{4}\) = \(\frac{75}{2 \times(-25)}\)
h
2
= \(\frac{-75}{2 \times 25}\) × 4 = -6 cm
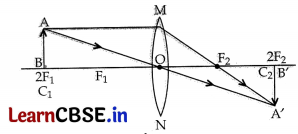
A real and inverted image of 6 cm is formed at 37.5 cm away from the lens on the other side of die object.
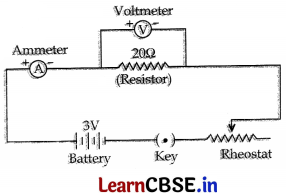
Question 27.
You have following material:
An ammeter (0 – 1A), a voltmeter (0 – 3V), a resistor of 20 Ω, a key, a rheostat, a battery of 3 V and seven connecting wires.
Using this material draw a labelled circuit diagram to study the dependence of potential difference (V) across a resistor on the current (I) passing through it.
Answer:
An ammeter (0-1 A), a voltmeter (0-3V), a resistor of 20 Ω, a key, a rheostat, a battery of 3 V and seven connecting wires.